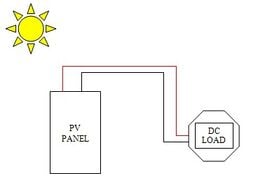
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons that will flow through an external electric circuit to the positive terminal. When a battery is connected to an external electric load, a redox reaction converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell.
Primary (single-use or "disposable") batteries are used once and discarded, as the electrode materials are irreversibly changed during discharge; a common example is the alkaline battery used for flashlights and a multitude of portable electronic devices. Secondary (rechargeable) batteries can be discharged and recharged multiple times using an applied electric current; the original composition of the electrodes can be restored by reverse current. Examples include the lead–acid batteries used in vehicles and lithium-ion batteries used for portable electronics such as laptops and mobile phones.
Batteries come in many shapes and sizes, from miniature cells used to power hearing aids and wristwatches to, at the largest extreme, huge battery banks the size of rooms that provide standby or emergency power for telephone exchanges and computer data centers. Batteries have much lower specific energy (energy per unit mass) than common fuels such as gasoline. In automobiles, this is somewhat offset by the higher efficiency of electric motors in converting electrical energy to mechanical work, compared to combustion engines.
Batteries in Photovoltaic Systems[edit | edit source]
Batteries store energy generated from panels for later use. This can be especially important in a photovoltaic system, as loads often need energy when the sun is not out, and some days have little sun, and some loads need more power than the panels put out. A battery can allow you to store energy for when the sun is not out or even decouple generation from use. For example, a panel outputting 100 W for 5 hours generates 500 Wh. A battery could then easily power a 200 W device for a couple of hours. This means that a small panel generating for a longer time can power a larger device for a shorter time.
Currently, storage is the largest technical block to renewable energy penetration in the market. Battery technology continues to progress but significantly lags behind the progress of photovoltaic technology. As with all aspects of photovoltaic system design, many options of batteries exist to meet specific needs. These types must be chosen to fit the energy needs of the user, system voltage, lifespan, costs, and other factors.
Gallery[edit | edit source]
- Some batteries in Appropedia Projects
-
Trojan Deep Cycle Lead Acid Battery
-
Deep cycle lead acid battery with a home made plastic terminal cover.
-
AGM sealed battery
-
Battery bank at CCAT MEOW
-
Car battery. Not ideal for renewable energy systems.
-
Battery terminal connector.
-
Lead acid 6 cell batteries.
-
MightyMax battery for CCAT solar bug out box
-
VRLA battery
-
Triple A Lithium Ion rechargeable battery
-
NiMH battery for How to Build a Mechanically Powered Battery Charger for LED Lighting
-
3.4Ah Battery Load Profile Procedure
-
Battery and system at Bayside Park Farm solar hot water/Previous version
-
Sealed deep cycle from Solar-charged lawnmower
-
From CCAT MEOW 2015
-
AGM battery from Treadmill-a-volt treadmill powered charging
-
Lithium Iron Phosphate from 535 The Last Airsensor remote air quality monitoring station
Instances[edit | edit source]
This gallery shows project instances on Appropedia that contain the keywords 'battery'.