Electrolysis is a promising option for carbon-free hydrogen production from renewable sources. It is quite an old technology that uses power supply to split water molecule into hydrogen and oxygen. This reaction takes place in a unit called an electrolyzer. Electrolyzers can range in size from small, appliance-size equipment that is well-suited for small-scale distributed hydrogen production to large-scale, central production facilities that could be tied directly to renewable energy sources.[1]There are different electrolyzers available in the market. Some popular electrolyzers are discussed below.
Alkaline Electrolyzers[edit | edit source]
Alkaline water electrolysis is old technology but this is one of the easiest, simplest and suitable methods for hydrogen production. Alkaline electrolyzer decomposes water at the cathode to hydrogen and hydroxide ion (OH-). The latter migrates through the electrolyte and a separating diaphragm, discharging at the anode liberating the O2. The electrolyte is an aqueous solution containing either NaOH or KOH with a typical concentration of 20–40 wt. %.[2] Although, alkaline electrolyzers are very cost effective but they lack in efficiency in comparison to others electrolyzers and they have a limited operating temperature range, which can affect their performance in extreme temperatures.
Polymer Electrolyte Membrane (PEM) Electrolyzers[edit | edit source]
In PEM electrolyzers, the electrolyte is a gas-tight thin polymeric membrane with a cross-linked structure and strongly acid character due to the presence of functional groups of the sulfonic acid (SO3H) type. These groups are responsible for the proton (H+) conducting ability of the materials through the membrane.[3] At the anode, water is oxidized to produce oxygen, electrons, and protons. H+ circulate across the membrane to the cathode where they are reduced to producing hydrogen. The main drawback for the commercially available PEM electrolyzers is their high investment costs, mainly associated to the membranes and the metal noble-based electrodes.
Solid Oxide Electrolyzers (SOE)[edit | edit source]
Solid oxide electrolyzers, which use a solid ceramic material as the electrolyte that selectively conducts negatively charged oxide ions (O2-) at high temperatures. Steam is fed to the cathode, where water is reduced to produce hydrogen and the oxide ions (O2-) generated in the cathode pass through the solid electrolyte to the anode, where they recombine forming oxygen and closing the circuit with the released electrons. The main current obstacle for the industrial application of SOEs is the limited long-term stability of the electrolysis cells.[3] Some of the problems that have been identified are electrolyte aging and electrode deactivation.
Anion Exchange Membrane (AEM) Electrolyzers[edit | edit source]
AEM electrolyzer is the latest technology to produce green hydrogen. It eliminates the disadvantages of all the above mentioned electrolyzers.The electrolyzer we are going to use in our project is of AEM type. That’s why a detailed study of AEM is going to be presentated. The anode and the cathode are separated by a solid membrane whose function is to transport hydroxyl ions(OH-) from cathode to anode and to act as a barrier for electrons and gases that are produced by the electrochemical reaction. It is composed of a polymer backbone coupled with anion exchange functional group, typically quaternary ammonium ion-exchange group. The polymer matrix is responsible for mechanical and thermal stability, while the functional group is responsible for the ion exchange capacity and ionic conductivity.[4]
- Cathode Reaction: 2𝐻2𝑂(𝑙) + 2𝑒- → 𝐻2(𝑔) + 2𝑂𝐻- (𝑎𝑞)
- Anode Reaction: 2𝑂𝐻- (𝑎𝑞) → + 𝐻2𝑂(𝑙) + 2𝑒-
- Overall Reaction: 𝐻2𝑂(𝑙) → 𝐻2(𝑔) + 1⁄2𝑂2(𝑔)
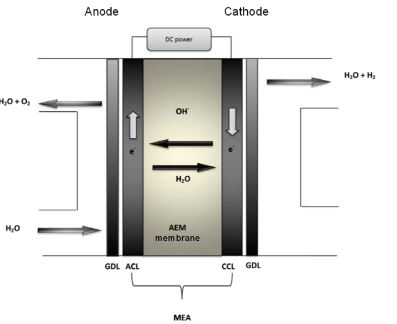
As depicted in Figure, the major component of the AEM electrolyzer consists of the gas diffusion layer (GDL), the electrocatalyst layer, and the AEM membrane, as well as the complete electrochemical process of water splitting for hydrogen production. Every AEM electrolyzer cell is divided into two half-cells with the AEM part in the middle. Each half-cell comprises several components, including the GDL and the electrocatalyst layer, either the anode catalytic layer (ACL) or the cathode catalyst layer (CCL). [5]
Literature review on "Open source solar powered AEM Electrolyzers"[edit | edit source]
Water electrolysis based on renewable energy for hydrogen production[6]
In 2017, Jun Chi and Hongmei Yu reviewed different water electrolysis processes to compare their feasibility in terms of their integration with solar photovoltaic (PV) system. The ions transfer mechanisms, operating characteristics, energy consumption, and industrial products of different water electrolysis techniques are discussed in this literature. The authors point out at the huge potential of solar power to produce clean green hydrogen.
Solar-powered regenerative PEM electrolyzer/fuel cell system[7]
In 2004, Daniel Shapiro, et.al designed a prototype to store hydrogen gas using PEM electrolyzer. Several tests were done to verify the performance of each component using the target system. PEM electrolyzer shows target current density of 1.0 A/cm2 at 65oC. Also this experiment find this system suitable to integrate with PV array by matching PEM electrolyzers actual load line with the maximum power points of typical commercial PV module (here, an ASE Americas ASE-50).
Optimization of solar powered hydrogen production using photovoltaic electrolysis devices[8]
In 2008, Thomas L. Gibson and Nelson A. Kelly discussed the optimization technique of solar powered hydrogen production by directly connecting PV module with electrolyzer. To reduce the overall cost and thus increase the efficiency of the system, PV-electrolysis system is simplified by eliminating charge controllers, storage batteries, and DC–DC converters. In this direct connection optimization (DCO) technique, the maximum solar-hydrogen efficiency was found 12.4%. Again in DC–DC converter optimization (DDO) in which a DC–DC converter is used, efficiency was recorded 10.6%.
Predicting efficiency of solar powered hydrogen generation using photovoltaic-electrolysis devices[9]
In 2009, Thomas L. Gibson, et. al developed a model to predict the efficiency of a PV-electrolyzer cell. The paper suggested increased efficiency by correctly matching the PV output voltage under load (the maximum power point voltage) to the operating voltage of the electrolysis system. For this experiment, PEM electrolyzer stack with 20 electrolysis cells in series powered by PV modules was optimized to give a hydrogen production efficiency of 12.4%.
Performance assessment of solar-powered high pressure proton exchange membrane electrolyzer: A case study for Erzincan[10]
In 2018, Hadi Ganjehsarabi conducted a comparative study to examine the effect of array size, number of stacks, and electrolyzer capacity on the performance of a solar PV-PEM electrolyzer. The model developed for PV-PEM electrolyzer provides a method for calculating the amount of hydrogen production for varying operating conditions. It this study it is found that hydrogen production rate is sensitive to cell operating temperature and pressure and the sensitivity of hydrogen production is obtained 8%.
Control and energy efficiency of PEM water electrolyzers in renewable energy systems[11]
In 2017, Joonas Koponen, et.al observed operation of a PEM electrolyzer supplied from a 5 kWp solar PV power plant. The dynamics and controllability of the PEM electrolyzer system were experimentally studied. In this investigation, when the hydrogen outlet pressure from the water electrolyzer stack was increased from 2.0 MPa to 4.0 MPa, the electrical energy consumption did not show any notable increase.
Techno-economic feasibility evaluation of a standalone solar-powered alkaline water electrolyzer considering the influence of battery energy storage system: A Korean case study[12]
In 2021, Haider Niaz, et. al simulated a standalone PV-Alkaline Water Electrolyzer (AWE) system to evaluate its economics and performance based on actual weather report in Korea. Four different configurations, among which case-1 without battery energy storage system (BESS) and case2-4, with BESS, but different battery control strategy are discussed in this literature. Although the result shows minimum levelized cost of hydrogen (LCOH) is obtained without any BESS, but the authors suggest a trade-off between the use of BESS and LCOH for continuous and reliable AWE operation.
Dynamic operation of water electrolyzers: A review for applications in photovoltaic systems integration[13]
In 2023, V.A. Martinez Lopez, et. al elaborately discussed the potentials and the barriers of integrating PV system with water electrolysis technology. The paper suggests a minimum solar to hydrogen efficiency of 10% needs to be maintained to make this system feasible and compatible. The main factors affecting the efficiency are unpredictable temperature and pressure.
Low cost hydrogen production by anion exchange membrane electrolysis: A review[14]
In 2017, Immanuel Vincent, et al. conducted a detailed study about the prospect of Anion Exchange Membrane (AEM) electrolysis technique. The major problem of PEM electrolysis technique, which is its high cost, can be altered with the use of low cost metal electrocatalysts in AEM, without degrading its ability to generate hydrogen gas. A brief discussion has been done for each of the components associated with AEM electrolysis techniques such as –membrane, ionomer, catalyst, MEA. This paper also summarizes the outcomes of different research work carried out on each component to evaluate its performance under various operating conditions. Finally, the shortcomings of this new technology is discussed along with some recommendations.
The promise of hydrogen production from alkaline anion exchange membrane electrolyzers[15]
In 2021, Changqing Li, et.al discussed about the basic components, progress and difficulties associated with the recently developed AEM electrolysis technique. Although Platinum Group Metal (PGM)-free electrocatalysts used in the AEM electrolysis can be cost effective in comparison of PEM electrolysis, but durability is the biggest concern for the development of PGM-free electrocatalysts. That’s why this paper suggest to use Ni-based compound only as cathode material, while using RuO2 as anode and by using this arrangement the cost of hydrogen is predicted 2.38 $ kg–1.
Comparative study of anion exchange membranes for low-cost water electrolysis[16]
In 2019, I.V. Pushkareva, et.al conducted a research experiment to verify the performance of various AEM membranes namely Sustanion (USA), A-201 (Japan) and AEMION™ (Canada) under identical conditions and using the same hardware. Experiments were carried out at different temperatures and electrolyte concentration. All the membranes exhibit fairly good performance but among all Sustanion based MEA shows the best results at all KOH concentrations and temperatures.
A review of alkaline solid polymer membrane in the application of AEM electrolyzer: Materials and characterization[5]
In 2021, Zulfirdaus Zakaria, et. al conducted a comparative study to find the characteristics of several Anion Exchange Membranes (AEM) namely Tokuyama A-201, Fumasep, Polysulfone, Poly (vinyl benzyl chloride), Polystyrene and Poly (vinyl alcohol). However, the author believes chemical and mechanical stability, cell durability is the matter of concern for the development of this new technology which can be the topic of further investigation and research.
An overview of water electrolysis technologies for green hydrogen production[17]
In 2022, S. Shiva Kumar and Hankwon Lim reviewed techno-commercial prospects of hydrogen generation by deeply analyzing recent development, research and challenges coming in the construction of components of electrolyzers. They recommend water electrolysis technology to be integrated with renewable energy sources like wind and solar to become the heart of the energy transition to meet net-zero challenges..
Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes[18]
In 2020, Behrooz Motealleh, et.al conducted experiment to verify the performance of Sustainion anion exchange membranes. The result shows stable performance of ~1 mV/h over 10,000 h of testing, resulting in a projected MEA lifetime of over 20 years. Also through impact and crossover testing, an improvement in performance is achieved by the addition of zirconia to the polymer matrix and mechanically reinforcing the membrane.
A standard electrolyzer test cell design for evaluating catalysts and cell components for anion exchange membrane water electrolysis[19]
In 2023, Abdulhai H. Faqeeh et.al created a standard AEM electrolyzer cell to perform tests for different electrocatalysts and membranes to compare their performance in a same test condition. Detailed experimental set up was discussed including specifications for each of the components. The performance of the water electrolyzer was tested using stainless steel fiber paper as the anode and 0.5 mg cm–2 Pt/C as cathode, deposited on two different cathode gas diffusion layers: carbon cloth and Sigracet carbon paper. Results show superior performance of carbon cloth in comparison with carbon paper. Again using the same experimental setup, tests were done to observe the effect of membrane by using Sustainion X37-50 membrane and Fumasep FAA-3-50 membrane. This electrolyzer showed excellent performance of 2.74 A cm–2 and 1.40 A cm–2 current density at 2.0 V and at 60◦C using carbon cloth and Fumasep FAA-3-50 and Sustainion X37-50 membranes, respectively.
Commercial Anion Exchange Membranes (AEMs) for Fuel Cell and Water Electrolyzer Applications: Performance, Durability, and Materials Advancement[20]
In 2023, Wei Keat Ng and his team analysed the performance of different commercially available AEMs in terms of their durability in AEM electrolyzers. While other literature surveys mainly focused on the properties of commercial AEMs themselves, this review emphasizes the significance of considering the interaction between the membrane and electrocatalyst and its impact on the overall performance of AEM-based systems.
Overview: State-of-the Art Commercial Membranes for Anion Exchange Membrane Water Electrolysis[21]
In 2021, Dirk Henkensmeier and his team presented a detail literature review about the characteristics and performance of commercially available AEM membranes under different working conditions. The summarized result give indication about all the important parameters including cell voltage, current density, operating temperature for varied range of AEM membranes, electrolytes, and ionomers. However, this study also suggest that the conductivity values cannot be exactly compared because a generally accepted standardized testing protocol that allows reliable comparison of different materials available does not exist.
Techno-economic modelling of AEM electrolysis systems to identify ideal current density and aspects requiring further research[22]
In August 2023, Laura J. Titheridge, et.al analyzed the AEM electrolysis techniques’ feasibility by estimating the levelized cost of hydrogen (LCOH). At an optimum current density of 1.38 A cm2, LCOH was found $5.79/kg, which is far more than the target of The U.S. Department of Energy (cost targets of < $2 kg-1 for end uses and < $1 kg-1 for industrial and bulk power generation uses). So to lower the LCOH some economic and performance strategies are proposed in this paper.
Investigation of Performance of Anion Exchange Membrane (AEM) Electrolysis with Different Operating Conditions[23]
In 2023, Adam Mohd Izhan Noor Azam and his team created an experimental set up to evaluate the performance of AEM electrolyzers under various operating conditions. The performance of the electrolyzer was examined by varying electrolyte concentration, electrolyte flow rate and temperature. By experimental results it is found that temperature and electrolyte concentration greatly affect the electrolysis efficiency while electrolyte flow rate has a little less impact compared to the others. The optimal conditions for the operation of the AEM electrolysis, was found as follows: 2 M KOH liquid electrolyte, a temperature of 60◦C, and a flow rate of 9 mL/min. Hydrogen production of 61.13 mL/min is achieved with an energy consumption of 48.25 kW.h/kg and an energy efficiency of 69.64%.
References[edit | edit source]
- ↑ F. Barbir, “PEM electrolysis for production of hydrogen from renewable energy sources,” Sol. Energy, vol. 78, no. 5, pp. 661–669, May 2005, doi: 10.1016/j.solener.2004.09.003.
- ↑ Md Mamoon Rashid, Mohammed K. Al Mesfer, Hamid Naseem, and Mohd Danish, “Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis,” Int. J. Eng. Adv. Technol. IJEAT, vol. 4, no. 3, pp. 80–93, Feb. 2015.
- ↑ 3.0 3.1 A. Ursua, L. M. Gandia, and P. Sanchis, “Hydrogen Production From Water Electrolysis: Current Status and Future Trends,” Proc. IEEE, vol. 100, no. 2, pp. 410–426, Feb. 2012, doi: 10.1109/JPROC.2011.2156750.
- ↑ R. Biga, “Experimental characterization of a pre-commercial Anion Exchange Membrane electrolyzer and its techno-economic prospects for industrial-scale hydrogen production,” laurea, Politecnico di Torino, 2021. Accessed: Sep. 24, 2023. [Online]. Available: https://webthesis.biblio.polito.it/19981/
- ↑ 5.0 5.1 Z. Zakaria and S. K. Kamarudin, “A review of alkaline solid polymer membrane in the application of AEM electrolyzer: Materials and characterization,” Int. J. Energy Res., vol. 45, no. 13, pp. 18337–18354, 2021, doi: 10.1002/er.6983.
- ↑ J. Chi and H. Yu, “Water electrolysis based on renewable energy for hydrogen production,” Chin. J. Catal., vol. 39, no. 3, pp. 390–394, Mar. 2018, doi: 10.1016/S1872-2067(17)62949-8.
- ↑ D. Shapiro, J. Duffy, M. Kimble, and M. Pien, “Solar-powered regenerative PEM electrolyzer/fuel cell system,” Sol. Energy, vol. 79, no. 5, pp. 544–550, Nov. 2005, doi: 10.1016/j.solener.2004.10.013.
- ↑ T. L. Gibson and N. A. Kelly, “Optimization of solar powered hydrogen production using photovoltaic electrolysis devices,” Int. J. Hydrog. Energy, vol. 33, no. 21, pp. 5931–5940, Nov. 2008, doi: 10.1016/j.ijhydene.2008.05.106.
- ↑ T. L. Gibson and N. A. Kelly, “Predicting efficiency of solar powered hydrogen generation using photovoltaic-electrolysis devices,” Int. J. Hydrog. Energy, vol. 35, no. 3, pp. 900–911, Feb. 2010, doi: 10.1016/j.ijhydene.2009.11.074.
- ↑ H. Ganjehsarabi, “Performance assessment of solar-powered high pressure proton exchange membrane electrolyzer: A case study for Erzincan,” Int. J. Hydrog. Energy, vol. 44, no. 20, pp. 9701–9707, Apr. 2019, doi: 10.1016/j.ijhydene.2018.12.007.
- ↑ J. Koponen, A. Kosonen, V. Ruuskanen, K. Huoman, M. Niemelä, and J. Ahola, “Control and energy efficiency of PEM water electrolyzers in renewable energy systems,” Int. J. Hydrog. Energy, vol. 42, no. 50, pp. 29648–29660, Dec. 2017, doi: 10.1016/j.ijhydene.2017.10.056.
- ↑ H. Niaz, M. M. Lakouraj, and J. Liu, “Techno-economic feasibility evaluation of a standalone solar-powered alkaline water electrolyzer considering the influence of battery energy storage system: A Korean case study,” Korean J. Chem. Eng., vol. 38, no. 8, pp. 1617–1630, Aug. 2021, doi: 10.1007/s11814-021-0819-z.
- ↑ V. A. Martinez Lopez, H. Ziar, J. W. Haverkort, M. Zeman, and O. Isabella, “Dynamic operation of water electrolyzers: A review for applications in photovoltaic systems integration,” Renew. Sustain. Energy Rev., vol. 182, p. 113407, Aug. 2023, doi: 10.1016/j.rser.2023.113407.
- ↑ I. Vincent and D. Bessarabov, “Low cost hydrogen production by anion exchange membrane electrolysis: A review,” Renew. Sustain. Energy Rev., vol. 81, pp. 1690–1704, Jan. 2018, doi: 10.1016/j.rser.2017.05.258.
- ↑ C. Li and J.-B. Baek, “The promise of hydrogen production from alkaline anion exchange membrane electrolyzers,” Nano Energy, vol. 87, p. 106162, Sep. 2021, doi: 10.1016/j.nanoen.2021.106162.
- ↑ I. V. Pushkareva, A. S. Pushkarev, S. A. Grigoriev, P. Modisha, and D. G. Bessarabov, “Comparative study of anion exchange membranes for low-cost water electrolysis,” Int. J. Hydrog. Energy, vol. 45, no. 49, pp. 26070–26079, Oct. 2020, doi: 10.1016/j.ijhydene.2019.11.011.
- ↑ S. Shiva Kumar and H. Lim, “An overview of water electrolysis technologies for green hydrogen production,” Energy Rep., vol. 8, pp. 13793–13813, Nov. 2022, doi: 10.1016/j.egyr.2022.10.127.
- ↑ B. Motealleh, Z. Liu, R. I. Masel, J. P. Sculley, Z. Richard Ni, and L. Meroueh, “Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes,” Int. J. Hydrog. Energy, vol. 46, no. 5, pp. 3379–3386, Jan. 2021, doi: 10.1016/j.ijhydene.2020.10.244.
- ↑ A. H. Faqeeh and M. D. Symes, “A standard electrolyzer test cell design for evaluating catalysts and cell components for anion exchange membrane water electrolysis,” Electrochimica Acta, vol. 444, p. 142030, Mar. 2023, doi: 10.1016/j.electacta.2023.142030.
- ↑ W. K. Ng, W. Y. Wong, N. A. H. Rosli, and K. S. Loh, “Commercial Anion Exchange Membranes (AEMs) for Fuel Cell and Water Electrolyzer Applications: Performance, Durability, and Materials Advancement,” Separations, vol. 10, no. 8, Art. no. 8, Aug. 2023, doi: 10.3390/separations10080424.
- ↑ D. Henkensmeier, M. Najibah, C. Harms, J. Žitka, J. Hnát, and K. Bouzek, “Overview: State-of-the Art Commercial Membranes for Anion Exchange Membrane Water Electrolysis,” J. Electrochem. Energy Convers. Storage, vol. 18, no. 024001, Aug. 2020, doi: 10.1115/1.4047963.
- ↑ L. J. Titheridge and A. T. Marshall, “Techno-economic modelling of AEM electrolysis systems to identify ideal current density and aspects requiring further research,” Int. J. Hydrog. Energy, Aug. 2023, doi: 10.1016/j.ijhydene.2023.08.181.
- ↑ A. M. I. Noor Azam et al., “Investigation of Performance of Anion Exchange Membrane (AEM) Electrolysis with Different Operating Conditions,” Polymers, vol. 15, no. 5, Art. no. 5, Jan. 2023, doi: 10.3390/polym15051301.
