
Currently, the majority of electronic waste is disposed of in a landfill. The remainder is processed in an energy inefficient manner, where only small amounts of the available metals are recovered. This is a problem for a number of reasons.
- This waste contains recoverable trace amounts of precious metals, and larger quantities of a variety of other metals and alloys, especially copper, aluminium and steel.
- Electronic waste contains high concentrations of heavy metals, brominated flame retardants, and other plastic additives that have proven to have adverse effects on humans. See Electronic waste for more information.
- The volume of electronic waste produced is high, and growing fast.[1] In 2005, the United States alone disposed of at least 1.5 million tons of electronic waste, and at most one quarter of that was recycled.[2] The situation is similar in Canada, with more than 71 000 tonnes of waste being disposed of in 2005, with just 26% of that being recycled.[3] In the province of Ontario, Canada, the percentage recycled in 2004 was just 2%.[4]
- Discarding recoverable metals is energy inefficient. The energy savings possible by recovering metals are high when compared to mining new material. The energy savings for aluminum is 95%, copper 85%, steel 74%, lead 65%, and zinc 60%. Recovering other materials such and paper and plastics can reduce energy usages by 64% and 80%, respectively.[5] The worst case scenario eliminates only the ore extraction and much of the transportation steps, which are both energy intensive.
Materials involved[edit | edit source]
There are a wide variety of materials involved in the multitude of items that can be classified as electronic waste. Each component adds to the complexity of any recycling effort. Below is an exploration of a number of the most common components, by weight.
Printed circuit boards[edit | edit source]
A printed circuit board, or PCB, is the piece of hardware that acts as a base and provides electrical connections to the mounted components. They are present in almost all types of electronic waste, including cellphones, computers, TVs, and printers. A PCB is made up of a number of components. Each step of the productions process is outlined below, to give an idea of the variety of materials involved.
FR-4 is the most common base material[6] for printed circuit boards. FR-4 is an abbreviation of Flame Retardant 4, referring to its flame resistance and self-extinguishing properties. It is a brittle material formed by hardening a woven fibreglass sheet with an epoxy resin, usually created from ethylene clorohydrin and bisphenol-A.[7] To give it the aforementioned self extinguishing properties, a brominated flame retardant is incorporated in the epoxy. Some flame retardants can be incorporated at the molecular level, like TBBPAW.[6] It can be easily coloured, with common colours including green, blue, red, and black.
The type glass used to create the fibreglass sheets is S-glass. Content ranges by weight are, 52-56% silicon dioxide, 16-25% calcium oxide, 12-16% aluminium oxide, 5-10% boron oxide, 0-2% sodium oxide or potassium oxide, 0-5% magnesium oxide, 0.05-0.4% iron oxide, 0-0.8% titanium oxide, and 0-1% fluorides.[6]
The FR-4 is layered with copper traces, and bonded with subsequent layers. Holes are drilled through the circuit board, and finally surface mount components are soldered to the board. Some of these printed circuit boards are rather complex. For instance, computer motherboards today have as many as eight layers.[8]
Surface mounted components[edit | edit source]
A wide variety of components are soldered onto printed circuit boards. A resistor is comprised of copper leads attached to a painted ceramic or carbon core.[9] Microchips are composed of small amounts of silicon, aluminium, and copper,[10] with plastic coatings. CPUs today have integral aluminium heatsinks and are built on their own printed circuit board. Capacitors contain a variety of metals and plastics, depending on size and type.
Casings[edit | edit source]
Most consumer electronic devices have plastic casings, such as a TV or a cell phone. Household appliances also have aluminium or steel cases. Other products, such as computer cases have both metallic and plastic components to them. Screws, buttons, switches, gears, and springs are sometimes present on or in cases.
CRT monitors[edit | edit source]
CRT, or cathode ray tubeW, monitors are currently being phased out in favour of LCDW monitors.
A CRT monitor consists of the electron gun and focusing equipment, a plastic casing and leaded glass. Lead is present to shield people from the x-raysW produced as a by-product of the electron acceleration. An analysis of the glass resulted in the following results, by weight, are 49.61% silicon dioxide, 24.17% lead oxide, 7.79% potassium oxide, 5.32% sodium oxide, 3.63% aluminium oxide, 2.99% strontium oxide, 2.30% calcium oxide, 1.96% barium oxide, 1.49% magnesium oxide, 0.58% zirconium oxide, 0.07% iron oxide and 0.07% phosphorous oxide.[11]
The lead and phosphorous oxide[12] are most difficult to deal with. Most of the lead (up to 98.6%) can be recovered through a pyrovacuum process, where high heat and low pressure allow the lead to be vaporized and later recovered, with the aid of carbon powder.[11]
Wire[edit | edit source]
Wire is a common element in electronic waste, as it has a method of connecting to a household wall socket, either through a power cord or charger. Internal wiring is common, especially in desktop computers and older audio and video equipment. It is mostly made of copper with plastic insulation, though the connection may be made from a number of different metals.
Batteries[edit | edit source]
Batteries are a staple for portable electronic devices, whether in lithium ionW, rechargeable NiCdW or NiMHW, AAA or AA alkaline cellW form. While batteries are electronic waste, they will not be covered in this analysis. There are numerous battery recycling programs; see [1][2][3].
Precious metals[edit | edit source]
Metals are removed by the melting down of all the materials. The metal is the very last material to melt and is easily separated.
Precious metals are used in electronics for their superior conductivity and resistance to oxidation. Gold is used as solder and connection pads where good electrical connections are paramount; this is easily seen on cell phone battery connections and on some audio cables.[13] Silver is used in batteries, solder and switches.[14] Palladium is used in capacitors and solder pads.[15]
A study undertaken by Cui, J. et Al., at the Norwegian University of Science and Technology showed that there are recoverable amounts of precious metals, such as silver, gold and palladium. For typical electronic scrap, they found and average of 2000 ppm silver, 1000 ppm gold and 50 ppm palladium. Outliers include printed circuit boards with 3300 ppm silver and cell phones with 210 ppm palladium.[16] This is significant, as this means that in the United States, using 2005 data,[17] these numbers show that there was up to 1500 tons of gold and 3000 tons of silver in the electronic waste that was disposed of in the U.S.A. in 2005.
Current solutions[edit | edit source]
"Solutions" is a misnomer for this category. Electronic waste processed in a manor shown below either does not recover usable materials, is selective of feedstock, or processes the electronic waste in a manner that has measured environmental effects. Most of the processes seen below have been conceived and implemented to meet one goal. Below are detailed the current or proposed solutions by recycling companies and researchers.
Shredding[edit | edit source]
In this process, commonly known as mechanical e-waste recycling, electronic waste is shredded by specialized equipment. An example of a shredder used in Kansas, U.S.A.[18] may be found here. This waste is then sorted mechanically, by magnetic fieldW or eddy currentW separators, or novel means such as vertical vibration separation.[19] This last method has been demonstrated to be effective for separating metals from plastics, especially copper.[19]
The metallic portion is then sold to a smelter, and the remaining shredded waste is landfilled.
Another product of this method is fine dust. Research has been conducted into possible uses of this dust. In a study conducted by Kakimoto, K et Al., it can be successfully incorporated into Portland cement, without the loss of strength, up to 30% by weight. An analysis of this dust found to that it was mainly silicon dioxide, calcium oxide, and aluminum oxide, with trace amounts of lead and copper.
This process is effective at separating metals from plastics, but fails to address the separation of low concentration metals from printed circuit boards and the effects the non-metallic waste will have on the environment.
Municipal incineration[edit | edit source]
Municipal incineration is simply just incinerating electronic waste with other household and business waste in a municipal incinerator. While many components of electronic waste have high usable energy content, this dilutes metal concentrations down even further.
Stewart, E. et Al. studied the emissions of incinerated computer components and monitored it for VOCs, SVOCs, dioxins, halogens, and metals. With a mass flow rate of 1.944 kg/h, their findings are as follows, with all weights listed being per dry standard cubic meter. The halogen chlorine at 7.9 mg/dscm, dichlorine 0.3 mg/dscm, bromine 13.1 mg/dscm, dibromine 17.8 mg/dscm. The metal concentrations were copper at 4600-7950 µg/dscm, lead 3790-4840 µg/dscm, antimony 790-1540 µg/dscm, cadmium 55-81 µg/dscm, manganese 19-52 µg/dscm, nickel 8-17 µg/dscm, barium 6-28 µg/dscm, arsenic 3-6 µg/dscm, chromium 3-4 µg/dscm, cobalt 0.7-2.3 µg/dscm, and beryllium 0.1-0.3 µg/dscm. Volatile organic compounds were present at bromobenzene 830-920 µg/dscm, tribromomethane 40-70 µg/dscm, bromomethane 20-190 µg/dscm, benzene 25-35 µg/dscm, dibromomethane 0-20 µg/dscm. Semi-volatile organic components were measured at naphthalene 1.2-3.3 µg/dscm, acetophenone 1.0-2.0 µg/dscm, chlorobenzene 0.4-1.4 µg/dscm, and dibenzofuran 0.3-0.8 µg/dscm.[20] These levels are all extremely small. It would be easy to dismiss them as insignificant, except for the scale of the trial - at less than 2 kg per hour, scaling up to multiple tonnes as hour would cause a proportionate increase in emissions. An output of such a wide range of harmful substances would then disqualify this method as a sustainable way of dealing with electronic waste.
It should be noted that the bromine in brominated flame retarded plastics and printed circuit boards were found to mainly produce a gas made of two dangerous compounds: hydrogen bromideW and bromobenzeneW.[21]
Pyrometallurgical recovery[edit | edit source]
This process is conducted by adding shredded electronic waste with high copper contents to copper ore concentrate and is then refined through the use of heat. Two examples of this, each recycling about 100 000 tonnes of electronic waste per year, are shown below.
At a copper smelter, in Quebec, Canada, the Noranda process is used. Copper ore concentrates at about 24% copper and shredded electronic waste are immersed in a liquid metal bath, at 1250 C. Oxygen enriched air (39% oxygen) is added. This converts iron, lead and zinc into oxides, which are removed with the silicon dioxide based slag. It is refined in an anode furnace, resulting in an alloy of 99.1% copper, with the remainder made up of silver, gold, platinum, palladium, selenium, tellurium and nickel.[16]
At the Ronnskar smelter in Sweden, a similar process is undertaken. Shredded electronic waste with low metal concentrations is added at the start of the process, while higher concentration e-waste is added later. Zinc is removed at an earlier step with slag and then separately refined. During the refining process, precious metals such as selenium, gold, silver and palladium, are removed at separate steps than lead, and nickel. This leaves a high purity copper as the final product.[16]
This process is relatively efficient, but is only useful for shredded electronic waste that meets the requirement of high metal content. Also, this process can not deal with aluminum, not does it account for the dioxins created due to the presence of brominated flame retardants.[16]
Open flame incineration[edit | edit source]
The waste that is shipped to India, China, and other Asian countries is burned, cut up, or dissolved in acids to recover metals, mainly copper that can be sold to scrap dealers. Components that have no resale value are dumped. Numerous written [4][5][6] and video accounts [7][8] are available.
It is believed that the majority of the waste treated in this way is exported from the United States of America, as it remains one of the few countries where the export of electronic waste remains legal. See Electronic waste legislation and practices.
Thermal depolymerization[edit | edit source]
Thermal depolymerization is a process in which thermal energy, under high pressure conditions and with the aid of water, is used to decompose organic molecules. It would, in theory, render plastics and epoxies present into usable oil. The resulting solids would have much higher concentrations of metals. The process is currently being used to convert turkey waste in to water, solid waste (mainly carbon) and oil.[22]
This process is effective in dealing with plastics, but offers no method of bromine recovery. Also the metal-laden slag generated would increase the metal content by weight, but would likely complicate the metal recovery process due to the high concentration of oxides. Therefore, no overall increase in recycling efficiency is expected. This process is widely lauded as the solution to organic waste, but does not suit the goal of maximum metal extraction.
Plasma arc gasification[edit | edit source]
Plasma arc gasification is the process of incinerating waste with the use of a superheated (up to 13 900 C at initiation), charged stream of air. This produces syngasW and molten glass, which includes all of the metals and other impurities. This process is in use on a small scale, worldwide, for recovering energy from municipal waste. This process is self sustaining, as only two thirds of the energy extracted from cooling the syngas is required to meet the energy requirements of the process. The remainder can be sold as electricity.
While this is an economically viable method of disposing of municipal waste, it is a very energy inefficient step in recovering metals. The oxide output contains the metals diffused throughout, rendering the metals harder to recover than the original waste.
Bioleaching[edit | edit source]
Bioleaching is the process of using bacteria and fungi to separate metals from electronic waste. It promises to be very energy efficient. Organisms such as Bacillus sp., Saccharomyces cereÍisiae, and Yarrowia lipolytica leach lead, copper, and tin from printed circuit boards when shredded into sub-millimeter sizes. Under ideal conditions, T. ferrooxidans and T. thiooxidans were able to mobilize at least 90% of the aluminum, copper, nickel, and zinc present.[23] One type of bacteria, C. violaceum, was able to leach gold from larger pieces of electronic waste (5 x 10 mm). It dissolved 14.9% of the approximately 10 mg of gold present as dicyanoaurate.[24]
The conditions required for the organisms to survive and leach these metals dictates that the electronic waste piece sizes are extremely small and have a low spatial density. This means that this process would be useful only for recovering metals from the dust generated by shredding.
Analysis[edit | edit source]
There are three benchmarks that any electronic waste recycling program can be judged by: how much of the internal or embodied energy can be reclaimed, how much of the metals can be removed, and how much impact the whole process has on the environment, including energy requirements and released hazardous materials.
It should be noted that reclaiming embodied energy is extremely difficult. The embodied energy in an item can be defined as the energy required to mine, produce, and process each raw material, to construct, and any shipping required. The only method to completely reuse the embodied energy is by disassembling an item and reusing each part for the original, or similar purpose. For example, a screw could be removed and used in another product, or a computer case could be re-used to hold a new computer. Therefore, the Energy category mainly refers to the potential of the energy released when incinerated.
| Process | Energy reuse | Metal reclamation | Environmental impact |
|---|---|---|---|
| Shredding | Low; non-metals are landfilled | High; metals sent to smelter | High |
| Municipal incineration | High, as this process aims to maximize this | None, unaddressed | All toxins either released through smoke or slag |
| Pyrometallurgical recovery | Low, non-metals not included in scope | High | Large energy requirements |
| Thermal depolymerization | High, waste transformed into useable materials | None, unaddressed | Low, efficient process, organic toxins decomposed |
| Plasma arc gasification | High, waste transformed into useable materials and power | None, unaddressed | Low, efficient process, toxins decomposed |
| Bioleaching | Low, non-metals are landfilled | High, many metals have 90% recovery rates | Very low energy process |
Proposed solution[edit | edit source]
From all of the processes outlined so far, an attempt at a comprehensive plan for the recovery of metals and ultimately the recycling of electronic waste can be made. The same three efficiency benchmarks will be used. These are the ultimate goals of this process; to recover the maximum amount of each metal present as possible, to use any energy present, and to have a minimal environmental impact by releasing no hazardous substances and using minimal power input.
Input[edit | edit source]
To optimize any process, inputs need to be quantified. Unfortunately, studies have produced varied results. This is to be expected, as different countries have differing amounts of each kind of electronic waste. Since there are likely millions of discrete consumer products with electronic parts, a definitive analysis of the average composition of electronic waste would require a lengthy survey including a large quantity of waste. So far, this has not been undertaken. The table below gives a good idea of what is to be expected.
Electronic waste composition by weight
| Source | Steel | Plastic | Copper | Oxides | Aluminum | Nickel | Tin | Lead | Zinc | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Nnorom, et al. | 7.7% | 30%1 | 10.9% | 40% | 1.7% | 2.5% | 3.9% | 1.5% | 1.1% | n/a |
| Widmer, et al. | 47.9% | 20.6%2 | 7% | 5.4% | 4.7% | n/a | n/a | n/a | n/a | 14.4%3 |
| Morf, et al.[25] | 36% | n/a | 4.1% | n/a | 4.9% | 10.3% | 2.4% | 2.9% | 5.1% | 34.3%4 |
1: Plastics 25%, bromine flame retarded plastics 5%.
2: Plastics 15.3%, bromine flame retarded plastics 5.3%.
3: Includes printed circuit boards, wood, concrete, ceramics and non-ferrous metal.
4: Includes all non-metals.
Process[edit | edit source]
When examining the processes currently in use, patterns emerge. Some processes ignore one of the main goals, while excelling at another. If these processes are used in concert, the end result is vastly improved.
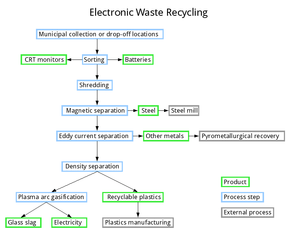
- When the waste is collected, batteries and CRT monitors are diverted to specialist recyclers. There are dedicated recycling companies for each of these items, so their expertise might as well be used.
- The remaining waste is shredded, with care that the dust is collected. There are a number of companies that produce dedicated electronic waste shredders – an example product line can be found here.
- The dust then undergoes a bioleaching process to remove metals. This process requires a considerable surface area, but this can be overcome by stacking surfaces vertically.
- The waste is subjected to magnetic separation, removing iron and steel. This is sent to a steel mill. This is a common process in single stream recyclingW. The steel is sold to a steel mill.
- Then waste then undergoes eddy current separation, which removes the majority of the remaining metal. Again, this is a common process in single stream recycling.
- Since the metals removed are varied, it is best if it undergoes a general-purpose refining process. The end product would be an alloy of a number of metals, provided none have been previously removed.
- Next, the waste is separated by density, allowing for some of the recyclable plastics to be removed and added to their respective recycling streams.
- The remainder, mainly plastic and printed circuit boards, undergoes either a thermal depolymerization or plasma arc gasification process to use as much of the internal energy present, either in the production of electricity or hydrocarbons. Both these processes generate more energy that they consume. Depending on specifics, they may be able to produce some or all of the power required for the rest of the plant.
An advanced exhaust gas system would need to be put in place. There have been a number of demonstrations of bromine recovery, either through the use of acidic and base gas scrubbers,[26] or just a sodium hydroxide scrubber.[27]
Economics[edit | edit source]
For the above process to work at all, the help of governments is needed. Legislation is required to make sure that all of the electronic waste a country produced is treated properly. When that happens, the remaining hurdle is cost. A processing plant that is essentially a single stream recycling plant and a plasma arc gasification plant together is expensive. Fortunately, this is much more feasible that having to process metal in-house.
All of the equipment required is off the shelf. Separation and shredding equipment is readily available, while the plasma arc gasification section would likely take some planning. There are currently a number of companies that have plants in the planning, developing or construction stages, so this technology is mature enough for a system that could be built in the near future.
Progress towards a sustainable future[edit | edit source]
Alternative materials and manufacturing[edit | edit source]
A number of alternatives have been proposed to the materials and processes currently used. These initiatives are detailed below.
- An alternative to FR-4 is a circuit board composed of chicken feathers with a soy-based epoxy. This product has been show to have superior electrical properties over FR-4 and drastically reduce the quantity and toxicity of chemicals used in the manufacturing process. It also has the added effect of using a material that is normally considered waste. An excellent article on the subject can be found here. If this technology becomes commonplace, this would have a massive effect on the composition of the average electronic waste.
- Lead free solder. The RoHSW Directive of the European Union currently specifies that products that are sold in their jurisdiction must contain no more than trace amounts of lead. Fortunately, this has had an impact on products available in North America, with the RoHS logo adorning many of the relevant electronic products in N.A. as well. This, along with the move to LCD based monitors and TVs will cause a large reduction in percentage lead composition of electronic waste.
- Elimination of halogens. The complications that halogens cause are many. An alternative to brominated flame retardants would have a very positive effect on the environmental release of halogens due to electronic waste.
References[edit | edit source]
- ↑ http://web.archive.org/web/20150721141747/http://www.epa.gov/epawaste/conserve/materials/ecycling/manage.htm Accessed Nov. 11, 2008
- ↑ http://web.archive.org/web/20150618105520/http://www.epa.gov/epawaste/conserve/materials/ecycling/docs/fact7-08.pdf Accessed Nov. 11, 2008
- ↑ http://www.ec.gc.ca/wmd-dgd/default.asp?lang=En&n=F3852FB1-1 Accessed Nov. 11, 2008.
- ↑ http://www.ene.gov.on.ca/en/news/2007/061201.php
- ↑ Nnorom, I. et Al., 2007, "Overview of electronic waste (e-waste) management practices and legislations, and their poor applications in the developing countries," Resources, Conservation and Recycling, 2008, (52), p. 5.
- ↑ 6.0 6.1 6.2 Coombs, C., 2001, Printed Circuits Handbook Fifth Edition, McGraw-Hill, New York, section 6.
- ↑ http://www.p-m-services.co.uk/how%27s_fr4_made_.htm Accessed Nov. 7, 2008.
- ↑ https://www.fudzilla.com/index.php?Itemid=37&id=7256&option=com_content&task=view Accessed Nov. 7, 2008.
- ↑ http://www.ecawa.asn.au/home/jfuller/electronics/resistors.htm Accessed Nov. 11, 2008.
- ↑ http://www.intel.com/education/makingchips/index.htm Accessed Nov. 11, 2008.
- ↑ 11.0 11.1 Chen, M. et Al., 2008, "Lead recovery and the feasibility of foam glass production from funnel glass of dismantled cathode ray tube through pyrovacuum process," Journal of Hazardous Materials, currently unprinted, p. 2.
- ↑ http://web.archive.org/web/20160307143842/http://avogadro.chem.iastate.edu/MSDS/P2O5.htm Accessed Nov. 12, 2008.
- ↑ http://www.azom.com/Details.asp?ArticleID=1899 Accessed November 24, 2008.
- ↑ http://www.edrsilver.com/i/pdf/2008-03-07_HR.pdf Accessed November 25, 2008.
- ↑ http://web.archive.org/web/20151121035928/http://www.stillwaterpalladium.com:80/electronics.html Accessed November 25, 2008.
- ↑ 16.0 16.1 16.2 16.3 Cui, J. et Al., 2007, "Metallurgical recovery of metals from electronic waste: A review," Journal of Hazardous Materials, 2008, (158), p. 3.
- ↑ http://web.archive.org/web/20150618105520/http://www.epa.gov/epawaste/conserve/materials/ecycling/docs/fact7-08.pdf p. 1, Accessed Nov. 11, 2008
- ↑ http://www.prlog.org/10046578-automated-waste-shredding-system-operational.html Accessed Nov. 12, 2008.
- ↑ 19.0 19.1 Mohabuth, N. et Al., 2006, "Investigating the use of vertical vibration to recover metal from electrical and electronic waste," Minerals Engineering, 2007, (20).
- ↑ Stuart, E. et Al., 2003, "Emissions from the Incineration of Electronics Industry Waste," IEEE, 2003, (03).
- ↑ Chien, Y. et Al., 1999, "Fate of bromine in pyrolysis of printed circuit board wastes," Chemosphere, 40, (4).
- ↑ http://www.sovereignty.org.uk/features/eco/zwaste2.html Accessed November 25, 2008
- ↑ Brandl, H. et Al., 1999, "Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi," Hydrometallurgy, 2001, (59).
- ↑ Cui, J. et Al., 2007, "Metallurgical recovery of metals from electronic waste: A review," Journal of Hazardous Materials, 2008, (158), p. 18.
- ↑ Morf, L. et a., 2006 "Metals, non-metals and PCB in electrical and electronic waste – Actual levels in Switzerland," Waste Management, 27.
- ↑ Boerrigter, H. et Al., "Bromine Recovery From The Plastics Fraction of Waste of Electrical and Electronic Waste (WEEE) With Staged Gasification," European Brominated Flame Retardant Industry Panel, 2002.
- ↑ http://www.flameretardants.eu/Objects/2/Files/R-2002_Conference_Geneve.pdf Accessed November 20, 2008.