
This STARS (Sexual and Reproductive Health and Rights) - Intrauterine Device (IUD) Insertion module allows nurses, midwives and clinical officers to become confident and competent in maintaining aseptic technique in sounding the uterus, loading the IUD in the sterile package, setting the gauge to the sounded depth, and gently inserting and deploying the IUD as part of copper and hormonal IUD insertion procedures for long-acting reversible contraception services performed in low to middle income countries.
Overview[edit | edit source]
Global Impact[edit | edit source]
An estimated 218 million women in low and middle income countries want to prevent pregnancy but lack access to effective birth control and/or comprehensive family planning and sexuality education.[1] The copper T380A and levonorgestrel-releasing (LNG) IUDs are safe, low-cost, and highly effective, long-term, reversible contraceptive methods that are appropriate for use by most women. Women should be provided the option of hormonal IUDs for long-term, reversible contraception because their better side effects profile may help increase rates of IUD use globally.[2] Copper IUDs can be utilized as emergency contraception in regions where emergency contraceptive pills are restricted or contraindicated.[3][4][5]
In 2017, approximately 295,000 women died during and following pregnancy and childbirth.[6] 94% of all maternal deaths occur in low to middle income countries with sub Saharan Africa accounting for two-thirds (196,000), Southern Asia accounting for nearly one-fifth (58,000), and South-Eastern Asia accounting for over 5% of global maternal deaths (16,000). To avoid maternal deaths, it is essential to prevent unwanted pregnancies.[7] Thus, all women, including adolescents, require access to safe, effective, and low-cost contraception.
Globally, 1 in 3 women experience sexual and/or physical abuse in their lifetime.[8] Gender-based violence (GBV) affects over 700 million women and girls globally at a systemic level (e.g., sexual slavery, human trafficking) and at an interpersonal level (e.g., rape, intimate partner violence).[9] In 2016, there were an estimated 4.8 million victims of sexual exploitation globally, with 99% of those being female.[10] The highest sexual exploitation rates were observed in Asia and the Pacific region followed by Central Asia and Europe. Copper IUDs can be inserted up to 14 days after unprotected intercourse and can prevent unwanted pregnancies in female survivors of sexual violence and sexual trafficking.[11]
A 2006 study reviewed 1,021 medical records of gender-based violence female survivors in DRC and observed an overall pregnancy rate of 6.1%.[12] The category of sexual violence is a significant predictor of resultant pregnancy rates which range from 2.1% for women experiencing gang rape to 5.5% for women experiencing rape not otherwise specified (NOS) to 14.3% for women experiencing combined gang rape and sexual slavery to 40.0% for women experiencing sexual slavery.
IUDs are under-used in many parts of the world, partially due to a lack of trained providers who are knowledgeable, skilled, and confident in delivering quality IUD services. To address this shortage, the World Health Organization recommends training medical personnel who are not physicians to insert IUDs to improve access to long-term and emergency contraception.[13]
Frugal and Open-Source 3D Printing Simulation Technologies for Copper and Hormonal IUD Insertion Skills Training[edit | edit source]
The frugal, low-tech Cervix and Vaginal Canal (with Augmented Feedback) Simulator is made of locally available, inexpensive materials and trains the learner to maintain aseptic technique in sounding the uterus. We require that learners have prior experience in pelvic examinations to address the inability of this simulator in teaching the performance of a pelvic speculum exam.
The 3D Printed Uterus Simulators offer different uterine depths so the learner can practice sounding the uterus and placing hormonal and copper IUDs at the uterine fundus.
The unfolding of the arms of the IUD is a critical step of the procedure. For copper IUDs, the practitioner inserts the IUD to the fundus and then pulls back on the inserter tube to permit the arms to spread upwards to the "T" position. For hormonal IUDs, the arms spread downwards to the "T" position so the practitioner must insert the IUD slightly past the os, and release the arms before advancing it to the fundus.
This module trains learners on how to insert copper and hormonal IUDs because using both types of IUDs does not happen frequently enough for practitioners or trainees to experience them in their practice, which can result in increased risk of patient morbidity, including but not limited to embedment of the IUD in the myometrium and perforation into the abdominal cavity.
The user's learnings will directly translate into clinical performance of aseptic technique for uterine sounding and proper insertion of hormonal and copper IUDs.
Syllabus[edit | edit source]
Phase 1: Knowledge Review[edit | edit source]
- Clinical Management of Rape (available in Arabic, English, French, Polish and Spanish)
- Adolescent Sexual and Reproductive Health Toolkit for Humanitarian Settings (available in Arabic, English, French, and Spanish)
- Pelvic Examination
- Intrauterine Device
- IUD Informed Consent and Pre-Insertion Counseling
- IUD Clinical Preparation
- Contraindications to Intrauterine Contraception
- Paragard Placement Training Guide (English only)
- Insertion Procedure for the Copper IUD Paragard
- Insertion Procedure for the 52mg LNG IUD Mirena
- AFP Long-Acting Reversible Contraception
- Difficult IUD Insertion
- Managing Pain with IUD Insertion
- IUD Insertion Self-Administered Readiness Test (coming soon!)
Healthcare services must play a key role in identifying survivors of gender-based violence (GBV) and linking GBV survivors to further support, including referrals to local or remote psychosocial support and case management services.[14]
Mental Health and Psychosocial Support for Survivors of Gender-Based Violence[edit | edit source]
- IASC Guidelines on Mental Health and Psychosocial Support in Emergency Settings
- IRC's WomenRise: A Gender-based Violence PSS Toolkit
- Mental health and gender-based violence Helping survivors of sexual violence in conflict – a training manual
Legal Protection for Survivors of Gender-Based Violence[edit | edit source]
Case Management for Survivors of Gender-Based Violence[edit | edit source]
Phase 2: Simulator Build[edit | edit source]
Phase 3: Skills Practice[edit | edit source]
Phase 4: Self-Assessment[edit | edit source]
- Training Logbook - Copper T380A IUD Insertion
- Training Logbook - Hormonal IUD Insertion
Phase 1: Knowledge Review[edit | edit source]
The learner should study the content of knowledge pages below and then take the practice quiz and readiness test. It is highly recommended that the learner be familiar with this content before proceeding to the skill pages.
- ACOG LARC Video Seriesː IUD Informed Consent and Pre-Insertion Counseling
- ACOG LARCː Video Seriesː IUD Clinical Preparation
- ACOG LARC Video Seriesː Contraindications to Intrauterine Contraception
- Paragard Placement Training Guide (English only)
- ACOG LARC Video Seriesː Insertion Procedure for the Copper IUD Paragard®
- ACOG LARC Video Seriesː Insertion Procedure for the 52mg LNG IUD Mirena®
- AFP Long-Acting Reversible Contraception: Difficult Insertions and Removals
- ACOG LARC Video Seriesː Difficult IUD Insertion
- ACOG LARC Video Seriesː Managing Pain with IUD Insertion
- IUD Insertion Self-Administered Readiness Test (coming soonǃ)
Phase 2: Simulator Build[edit | edit source]
- 3D Printed Uterus Simulators (in progress)
- Cervix and Vaginal Canal (with Augmented Feedback) Simulator (in progress)
Phase 3: Skills Practice[edit | edit source]
- Copper T380A IUD Insertion (coming soonǃ)
- Levonorgestrel-releasing (LNG) IUD Insertion (coming soon!)
Phase 4: Self-Assessment[edit | edit source]
- Training Logbook - Copper T380A IUD Insertion (coming soon!)
- Training Logbook - Hormonal IUD Insertion (coming soon!)
The self-assessment framework includes:
- Step-by-step procedural checklists to confirm proper insertion technique for copper and hormonal IUDs (including identification of uterine depths that are contraindicated for IUD insertion)
- Augmented feedback in the form of an auditory tone and/or light signal to alert the user if the sterile metal uterine sound touches the vaginal sidewalls, metal pelvic speculum blades, or tenaculum
- Timed trials to verify that the arms of the copper IUD are not bent for more than 5 minutes to ensure the arms will open properly in accordance with the manufacturer's instructions for use
- Timed trials to verify that the practitioner has waited at least 10 seconds to allow the horizontal arms of the hormonal IUD to open and regain its T-shape in accordance with the manufacturer's instructions for use
- Visual inspection to verify uterine depth and proper placement of copper and hormonal IUDs at the uterine fundus
The two simulators are designed to include a mechanism for targeted feedback which enables the user to: ensure they are practicing the appropriate skills; modify their performance to improve competence; and determine when they have practiced to a sufficient level of mastery to perform the procedure in a patient.
To enable the user to ensure they are practicing the appropriate aseptic ("no touch") technique with the metal uterine sound, the Vaginal Canal with Augmented Feedback Simulator provides augmented feedback (in the form of an auditory tone and/or light signal) to the learner when the sterile uterine sound touches the vaginal sidewalls, metal pelvic speculum blades, or tenaculum.
To enable the user to ensure they are practicing the appropriate uterine sounding, clinical decision-making, and IUD insertion skills, the 3D Printed Uterus Simulators provide a range of uterine depths (5.0 - 10.0 cm).
- A copper IUD should not be inserted if the depth of the uterus is less than 6.0 or more than 9.0 cm but this may vary depending on the specific device so the learner must always review the manufacturer's instructions for use beforehand.[15]
- A hormonal IUD should not be inserted if the uterine depth is less than 6.0 cm or more than 10.0 cm but this may vary depending on the specific product so the learner should review the manufacturer's directions for use beforehand.[16]
After completion of the simulated procedure, the learner can remove the lid of the 3D Printed Uterus Simulator to visually inspect and confirm the uterine depth and proper placement of the hormonal and copper IUD at the uterine fundus.
Innovation[edit | edit source]
The STARS - Intrauterine Device (IUD) Insertion module is better and more effective than traditional approaches available in LMICs to nurses, midwives, and clinical officers.
Augmented Feedback[edit | edit source]
The team is not aware of any IUD Insertion Simulator that provides augmented feedback to alert the learner if the sterile metal uterine sound touches the vaginal sidewalls, metal pelvic speculum blades, or tenaculum.
Higher Fidelity[edit | edit source]
The ZOE® Gynecological Skills Trainer provides one clear uterus for unblinded IUD insertion training.[17] The 3D Printed Uterus Simulators offer a range of uterine depths for blinded IUD insertion training which more accurately reflects the real clinical scenario for this procedure.
Reusable[edit | edit source]
Unlike single-use fruit-based simulators, the 3D Printed Uterus Simulators and Cervix and Vaginal Canal (with Augmented Feedback) Simulator are fully reusable with an indefinite lifespan which minimizes their per use cost.
Eco-Friendly[edit | edit source]
The 3D Printed Uterus Simulators are made from polylactic acid (PLA), a biorenewable, biodegradable, and minimally off-gassing thermoplastic.[18][19][20][21][22][23]
Easy to Print[edit | edit source]
The 3D Printed Uterus Simulators are open source and can be locally made on open-source, open filament, user friendly, single extruder desktop 3D printers that print polylactic acid, a low-cost and easy to print plastic.[24][25][26][27][28][29]
Unlike many 3D printed models, all of the module's 3D Printed Uterus Simulators are designed to:
- print without support material, rafts or brims
- require no cleaning, sanding, gluing, priming, painting, dipping, coating, smoothing, polishing, or any post-processing
- not require any non-3D printed parts, and
- be ready for use right out of the 3D printer.
Evaluation[edit | edit source]
This module's 3D Printed Uterus Simulators, and Cervix and Vaginal Canal with Augmented Feedback Simulator were originally demonstrated at three GSTC MIT Solveathon Workshops held between July 28, 2020 to September 19, 2020. Below is a description of how we developed and iterated our original prototype design following user testing that started on October 5, 2021.
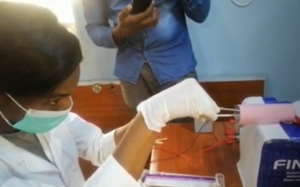
- The Team Co̟-Lead's comments after user testing in Nigeria was that "the nurses were so excited" and "this is a good invention." The feedback included: "It's important for learners to learn how to use one hand to hold the tenaculum to stabilize the cervix and use the other hand for the procedure. This is why simulation training on simulators is important because the learner can practice this skill multiple times before they perform this procedure on a live patient."
- Our user testing was conducted in Uganda with the Team Co-Lead and a senior colleague who is a Medical Officer with 20 years of clinical experience with maternity wards. Their feedback on our 3D Printed Uterus Simulators and Cervix and Vaginal Canal (with Augmented Feedback) Simulators is they are "way cheaper than the mannequin and easy to do." These users stated that our simulators simulate the features of navigating a small space, blinded insertion, and different uterine depths required for IUD insertion.
- Our user testing in Uganda showed that the 3D Printed Uterus Simulators required a base or stand to be flush with the angled Vaginal Canal with Augmented Feedback Simulator. It was useful to place a roll of masking tape and a notebook underneath the 3D Printed Uterus Simulator to position it to line up with the Vaginal Canal in the support pillars of the Gynecologic Simulator without the angled plane. We are working on creating a cardboard or 3D printed stand for the 3D Printed Uterus Simulators.
- The Ugandan Team Co-Lead suggested that alternatively we could switch to a flexible 3D printed filament to permit the learner to use the tenaculum to gently grasp the simulated cervix which could be incorporated as part of the 3D Printed Uterus Simulators.
- The users in Uganda observed that the curved metal uterine sound can lift off the lid of the 3D Printed Uterus Simulators. Our team has now increased the depth/height of the 3D Printed Uterus Simulators to permit adequate clearance with a curved metal uterine sound.
- Our user testing in Uganda and Nigeria found that the copper IUD does not remain at the fundus in the 3D Printed Uterus Simulators because the uterine width is greater than the width of the copper IUD when the arms are unfolded. Our team has decreased the width of the uterus to 3.1 mm in the 7.0 cm 3D Printed Uterus Simulators to match the length of the unfolded (outstretched) copper IUD arms to prevent the copper IUD from dropping down in the uterus.

- Our user testing in Uganda made their cervix models using a bread dough recipe and found it was too soft to stay in place during insertion. To address this, we will work on refining the homemade clay recipe to make stiffer clay. Alternatively, one Ugandan user also suggested that it might be better if we incorporated the cervix model into the 3D Printed Uterus Simulators.
- Our user testing in Nigeria found that the heavier tenaculum instrument sometimes inadvertently removed part of the clay cervix so they recommended using a less traumatic Allis tissue clamp. The learners were able to use the simulators to gain confidence and competence in providing gentle traction on the cervix with the Allis tissue clamp.
- Our Team Co-Lead in Uganda informed us that a sponge-holding metal forceps is preferred for grasping the cervix during real procedures because this instrument is less traumatic to the cervix compared to a tenaculum. Our user testing in Uganda demonstrated that it was possible to use a sponge-holding metal forceps to grasp the cervix model made of homemade clay.
- Our user testing in Uganda showed that the pink food coloring of the homemade clay leaves a visible residue on the metal uterine sound to permit the learner to measure the depth of the 3D Printed Uterus Simulator.
- Our user testing identified that using a oil-based (stiffer) clay for the cervix model provides better stability during the passage of instruments and IUDs into the 3D Printed Uterus Simulators.
- Our user testing showed that a sponge with a central perforation could also potentially be used to simulate the cervix, mounted on the vertical pegs of the 3D Printed Uterus Simulators, grasped by a tenaculum, and permit the insertion of the uterine sound in the cervical os. Our team purchased and tested several brands of makeup sponges but found these were too dense to allow the creation of the cervical os to permit the uterine sound and IUD to pass through into the cervix. Our user testing found that less dense kitchen sponges were better but we may need to redesign the 3D Printed Uterus Simulator to better retain the lighter sponge in place during the procedure.

- Our user testing in Uganda and Nigeria showed that the Vaginal Canal with Augmented Feedback Simulator could be constructed locally and would be functional.
- Our user testing identified that it is difficult to insert a curved metal uterine sound through a narrow (4.5 cm) diameter Vaginal Canal with Augmented Feedback Simulator that is flush with the 3D Printed Uterus Simulators. We purchased a malleable metal uterine sound that can be straightened.
- User testing was conducted in Nigeria with two nurses, a Medical Officer and our Team Co-Lead, who were able to manually straighten the curved metal uterine sound and successfully insert copper IUDs using the Vaginal Canal with Augmented Feedback that was flush with the 3D Printed Uterus Simulators.
- The users in Uganda demonstrated that it is possible to insert a metal curved uterine sound into the 3D Printed Uterus Simulators and maintain aseptic ("no touch") technique when the Vaginal Canal with Augmented Feedback Simulator is angled at 45 degrees using the support pillars and base of the Gynecologic Simulator and elevating the 3D Printed Uterus Simulators so the cervical os is centered on the Vaginal Canal.
- Our user testing confirmed that the Vaginal Canal with Augmented Feedback circuit would be activated if the metal uterine sound contacted the vaginal sidewalls (lined with aluminum foil), stainless steel Pederson pelvic speculum blades, or metal tenaculum grasping the cervix sponge model.
- To maximize learner safety, the Vaginal Canal with Augmented Feedback Simulator assembly instructions now include clipping the alligator clip directly to the metal uterine sound to avoid having any length of bare wire exposed in a potentially live electrical circuit.
Design for Extreme Accessibility in Low Resource Settings[edit | edit source]
This module applies user-centered, reproducible, and accessible design choices to maximize adoption in resource-constrained settings.
User-Centered Design[edit | edit source]
Our intended users are nurses, midwives, and clinical officers, working in primary health care facilities and mobile units in resource-constrained settings and who have prior experience in conducting pelvic examinations.
The traditional in-person teaching approach to IUD insertion available to our users requires access to courses, trainers, and expensive mannequins. Practitioners in resource-constrained settings may not have access to costly mannequins for training. This module provides instructions to the learner on how to build the non-3D printed components of the simulators out of readily available, low cost materials.
Learners need access to safe simulation training. To maximize safety and minimize the risk of the learner touching any conductive material in a live electrical circuit during simulation training:
- we added an extra insulated alligator clip instead of using a non-insulated metal wire to attach to the metal uterine sound
- we will be revising the simulator build and use instructions to re-emphasize and explain the safety rationale for the mandatory use of gloves during training.
Nurses, midwives, clinical officers, and medical officers in LMICs have busy work schedules and typically do not have a technical background. We tailored the design of this prototype training module to meet their training needs by designing the 3D Printed Uterus Simulators and Cervix and Vaginal Canal (with Augmented Feedback) Simulators to not require any tools, specialized equipment, or technical expertise to build, install, operate and maintain these simulators within the intended place of use.
This module is designed to reach and support as many nurses, midwives, and clinical officers in LMICs as possible. This self-assessed module does not require access to training centers, course workshops, teachers, or expensive mannequins.
Adoption in Resource-Constrained Settings[edit | edit source]
We considered adoption in low resource settings in the design of our surgical training module by tailoring the design of both simulators to be made locally and be fully reusable which maximizes their lifespan in the intended place of use.
Over 4 billion people do not have access to the Internet.[30] The penetration of high-speed Internet connectivity (broadband, 3G, or better mobile connections) is less than 30% in rural regions.[31] Smartphones only make up 50% of total connections in sub Saharan Africa.[32] In 2021, nearly 711 million people were in extreme poverty, which is defined as living on less than $1.90 per day.[33] To enhance adoption of this surgical training module in low resource settings, we:
- Designed cost-saving 3D Printed Uterus Simulators and Cervix and Vaginal Canal (with Augmented Feedback) Simulators to be fully reusable to minimize the per use cost and maximize their lifespan in the place of use
- Created frugal Cervix and Vaginal Canal (with Augmented Feedback) Simulators that are made from low cost, locally available materials
- Innovated Nulliparous and Parous Cervix Models that can be made using locally obtained modelling clay or easily made with a homemade recipe using flour, food colouring, salt, and water, and
- Developed a prototype Appropedia module which does not require the downloading of a mobile app, creation of an account, inputting of a username and password, or paying journal or other subscription fees to access the training content.
To help ensure that practitioners from anywhere in the world will be able to engage with the simulators without barriers, the 3D Printed Uterus Simulators have the uterine depths semi-engraved in the 6 official languages of the United Nations.
Reproducible Design[edit | edit source]
The module and simulators are designed to be fully reproducible in the intended place of use.
3D printing technology empowers the local, reliable, and automated manufacturing of a range of uterus models to permit high quality, standardized IUD insertion simulation training around the world. All of the model files and print settings for the 3D Printed Uterus Simulators will be open-source and available on Appropedia, can be locally reproduced on open-source, open filament desktop 3D printers using low-cost, biorenewable plastic, and are designed to be ready for use right out of the 3D printer. These reusable 3D Printed Uterus Simulators that offer a range of uterine depths are designed to substantially reduce simulator costs, simplify the simulator build, minimize the simulator assembly time, and accelerate the learning process for greater convenience for the learner.
On-site access to a 3D printer is not required for the learner. Only one 3D printer is required within a country. The open-source 3D files can be emailed to any 3D printing organization anywhere.[34] The 3D printed simulation models can be picked up by the learner or delivered anywhere across the country by motorcycles, all-terrain vehicles, trucks, or airplanes within 1-2 days.[35]
This module does not require access to teachers, mannequins, or patients. This module uses:
- A Cervix and Vaginal Canal (with Augmented Feedback) Simulator that is made from readily available, low-cost materials, and
- Locally reproduced 3D Printed Uterus Simulators for copper and hormonal IUD insertion procedure skills training.
The demand for this module will be greatest in regions with little or no access to the Internet, smartphones, or grid electricity. When possible, we will be providing step-by-step instructions and labelled images (instead of only videos) and publishing our self-assessment frameworks directly in Appropedia so the module content can be automatically translated into multiple languages and exported in pdf format for offline access.
Risks to Reproducibility[edit | edit source]
The primary risk to the reproducibility of our training module is access to the consumables, namely, the copper and hormonal IUDs. We will be developing local and international partnerships to provide IUDs and other related equipment (like reusable metal uterine sounds) at minimal cost to learners in LMICs.
We have developed local partnerships to manufacture and deliver 3D Printed Uterus Simulators at minimal cost for learners in Nigeria and Uganda.[34][35]
We will continue to refine and improve the instructions for building the Cervix and Vaginal Canal (with Augmented Feedback) Simulator using locally available, inexpensive materials for learners in LMICs.
Costs, Materials, Tools and Required Expertise[edit | edit source]
The total manufacturing costs of one basic trio set of 3D printed PLA Uterus Simulators (with uterine depths of 5.0, 7.0, and 10.0 cm) is $5.69, $44.39, and $45.00 USD in Canada, Uganda, and Nigeria, respectively. However, these simulators can be re-used indefinitely by multiple learners which makes their per use cost very low.
The total cost of the reusable Cervix and Vaginal Canal (with Augmented Feedback) Simulator is $7.26 USD in Uganda and $43.20 USD in Nigeria. This simulator is made using readily available materials and locally available supplies outlined in the table below:
| Item | Quantity | Cost in USD in Nigeria | Cost in USD in Uganda |
|---|---|---|---|
| Light Pink Clay | 2 pieces weighing approximately 57 grams (2 ounces) | Locally made with flour, food colouring, salt, and water | $3.07 USD |
| Ruler | 1 | Readily available in place of use | Readily available in place of use |
| Pencil or Chopstick | 1 | Readily available in place of use | Readily available in place of use |
| Paper | 1 | Readily available in place of use | Readily available in place of use |
| Aluminum Foil | 1 piece with estimated minimum dimensions of 11.0 cm by 30.0 cm (size may vary) | Readily available in place of use | Readily available in place of use |
| Scissors | 1 | Readily available in place of use | Readily available in place of use |
| Tape | Multiple strips | Readily available in place of use | Readily available in place of use |
| Optional: Wire Stripper | 1 | Not used | Not used |
| Buzzer | 1 | $11.28 USD | Not used |
| Alligator Clips | Minimum 4 | $19.20 USD (each alligator clip costs $4.80 USD in Nigeria) | $4.19 USD |
| Battery | 1 | $12.72 USD | Covered above |
| Optional: Light-Emitting Diode (LED) or Light Bulb Lamp | 1 | Not used | Covered above |
No tools, specialized equipment, or technical expertise is required to build, install, operate and maintain the two simulators within the intended place of use.
Value for Money[edit | edit source]
This module offers significant value for money in comparison to existing reusable approaches for IUD insertion training.
Both the 3D Printed Uterus Simulators and Cervix and Vaginal Canal (with Augmented Feedback) Simulator are fully reusable which minimizes the per use cost for learners.
The reusable ZOE® Gynecological Skills Trainer costs $2,695 USD.[17] The total cost of $51.65 USD ($44.39 + $7.26 USD) for the module's two fully reusable simulators in Uganda is over 52 times cheaper than the ZOE® Gynecological Skills Trainer, which only has 1 clear uterus for non-blinded IUD insertion training and does not provide augmented feedback if aseptic ("no touch") technique is not maintained.
By using locally made simulators, the learner also saves on customs dues, processing fees, and international shipping costs that would be incurred when purchasing the ZOE® Gynecological Skills Trainer which is not locally made.
This self-assessed module also permits the learner to save on the costs of course fees, travel, lodging, and missing work.
Locally Available Materials[edit | edit source]
The 3D Printed Uterus Simulators can be made locally with locally produced or imported plastic filament. On-site access to a 3D printer is not required for the learner. Only one 3D printer is required within a country. The open-source 3D files for the 3D Printed Uterus Simulators can be emailed to any 3D printing organization anywhere. The 3D printed simulation models can be picked up by the learner or delivered anywhere across the country by motorcycles, all-terrain vehicles, trucks, or airplanes within 1-2 days.[35]
The Cervix and Vaginal Canal (with Augmented Feedback) Simulator are made using readily available materials and supplies, including homemade clay, paper, aluminum foil, tape, alligator clips, buzzer, and 9 V battery.
Accessible Design[edit | edit source]
This self-assessed module does not require access to teachers, training centers and courses.
This Appropedia module is available in the 6 official languages of the United Nations and other languages to help ensure that surgical practitioners from anywhere in the world will be able to engage with the content without language barriers.
To help ensure that practitioners from anywhere in the world will be able to engage with the simulators without barriers, the 3D Printed Uterus Simulators have the uterine depths semi-engraved in the 6 official languages of the United Nations.
We will publish our self-assessment frameworks directly in the Appropedia module (instead of a downloadable pdf) to provide automatic translations of the Training Logbooks in multiple languages to learners around the world.
We will provide step-by-step instructions and labelled images (instead of only videos) so the module content can be available in multiple languages and exported for offline access. In the future, we can create line drawings of our graphics to save on color printing costs for learners in LMICs.
This module does not require the downloading of a mobile app, creation of an account, inputting of a username and password, or paying journal or other subscription fees to access the training content.
Follow-on[edit | edit source]
(Optional) After completion and practice to competency, the learner may wish to continue study with these STARS courses:
- STARS - Cervical Cancer Screening and Treatment for the estimated 604,000 women who were diagnosed with cervical cancer worldwide in 2020[36]
- STARS - Postpartum Hemorrhage for the estimated 14 million women who suffer from postpartum hemorrhage globally each year (coming soon!)[37]
- STARS - Obstetric Fistula Repair for the over 2 million women who suffer from the debilitating symptoms and social isolation from obstetric fistulas worldwide (coming soon!)[38]
References[edit | edit source]
- ↑ Family planning [Internet]. United Nations Population Fund; [cited 2021 Nov 28]. Available from: https://www.unfpa.org/family-planning.
- ↑ Ali M, Folz R, Farron M. Expanding choice and access in contraception: an assessment of intrauterine contraception policies in low and middle-income countries. BMC Public Health. 2019 Dec 19;19(1):1707. doi: 10.1186/s12889-019-8080-7. PMID: 31856766; PMCID: PMC6924003.
- ↑ Manual Inter-Agency Emergency Reproductive Health Kits for Use in Humanitarian Settings. 6th edition, 2019. https://www.unfpa.org/sites/default/files/resource-pdf/IARH-Kits-6th-Edition_Manual_English.pdf.
- ↑ https://en.wikipedia.org/wiki/Emergency_contraceptive_availability_by_country
- ↑ World Health Organization (WHO), United Nations Population Fund (UNFPA), United Nations High Commissioner for Refugees (UNHCR). Clinical management of rape and intimate partner violence survivors: developing protocols for use in humanitarian settings. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
- ↑ Trends in maternal mortality: 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization; 2019.
- ↑ Maternal Mortality [Internet]. World Health Organization; 2019 [cited 2021 Dec 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/maternal-mortality.
- ↑ World Health Organization. (2021, March 9). “Violence Against Women”. Retrieved from https://www.who.int/news-room/fact-sheets/detail/violence-against-women.
- ↑ Keyser L, Maroyi R, Mukwege D. Violence Against Women - A Global Perspective. Obstet Gynecol Clin North Am. 2022 Dec;49(4):809-821. doi: 10.1016/j.ogc.2022.08.002. PMID: 36328682.
- ↑ Global estimates of modern slavery: Forced labour and forced marriage International Labour Office (ILO), Geneva, 2017. ISBN: 978-92-2-130132-5.
- ↑ Thompson I, Sanders JN, Schwarz EB, Boraas C, Turok DK. Copper intrauterine device placement 6-14 days after unprotected sex. Contraception. 2019 Sep;100(3):219-221. doi: 10.1016/j.contraception.2019.05.015. Epub 2019 Jun 7. PMID: 31176689; PMCID: PMC7176316.
- ↑ Bartels SA, Scott JA, Mukwege D, Lipton RI, Vanrooyen MJ, Leaning J. Patterns of sexual violence in Eastern Democratic Republic of Congo: reports from survivors presenting to Panzi Hospital in 2006. Confl Health. 2010 May 5;4:9. doi: 10.1186/1752-1505-4-9. PMID: 20444265; PMCID: PMC2883538.
- ↑ Task sharing to improve access to family planning/contraception [Internet]. World Health Organization; [cited 2021 Nov 28]. Available from: http://apps.who.int/iris/bitstream/handle/10665/259633/WHO-RHR-17.20-eng.pdf;sequence=1.
- ↑ Murphy. M, Bourassa. A. (2021) ‘Gap Analysis of Gender-Based Violence in Humanitarian Settings: a Global Consultation’. Elrha: London.
- ↑ Johnson BA. Insertion and removal of intrauterine devices. Am Fam Physician. 2005 Jan 1;71(1):95-102. PMID: 15663031.
- ↑ Highlights of Prescribing Information [Internet]. Mirena. US Food and Drug Administration; [cited 2021 Dec 10]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021225s027lbl.pdf.
- ↑ 17.0 17.1 Gaumard Scientific. Zoe® S504.200 - Gynecological Skills Trainer [Internet]. Gaumard Scientific. Gaumard Scientific; 2021 [cited 2021 Nov 28]. Available from: https://www.gaumard.com/s504-200.
- ↑ Liu, K., Madbouly, S. A., Schrader, J. A., Kessler, M. R., Grewell, D. and Graves, W. R. (2015), Biorenewable polymer composites from tall oil-based polyamide and lignincellulose fiber. J. Appl. Polym. Sci., 132, 42592. doi: 10.1002/app.42592.
- ↑ Mills, C. A.; Navarro, M.; Engel, E.; Martinez, E.; Ginebra, M. P.; Planell, J.; Errachid, A.; Samitier, J. J. Biomed. Mater. Res. A 2006, 76A, 781.
- ↑ Auras, R.; Harte, B.; Selke, S. Macromol. Biosci. 2004, 4, 835.
- ↑ Chemical Composition and Toxicity of Particles Emitted from a Consumer-Level 3D Printer Using Various Materials. Qian Zhang, Michal Pardo, Yinon Rudich, Ifat Kaplan-Ashiri, Jenny P. S. Wong, Aika Y. Davis, Marilyn S. Black, and Rodney J. Weber. 2019 53 (20), 12054-12061. DOI: 10.1021/acs.est.9b04168.
- ↑ Emissions of Ultrafine Particles and Volatile Organic Compounds from Commercially Available Desktop Three-Dimensional Printers with Multiple Filaments. Parham Azimi, Dan Zhao, Claire Pouzet, Neil E. Crain, and Brent Stephens. 2016 50 (3), 1260-1268. DOI: 10.1021/acs.est.5b04983.
- ↑ Ultimaker. 3D printer emissions and indoor air quality -White paper. [Internet]. Utrecht (Netherlands): Ultimaker BV; 2019 Oct [cited 2021 Aug 17]. Available from: https://ultimaker.com/learn/3d-printer-emissions-and-indoor-air-quality.
- ↑ Prusa J. Open-Source 3D printers from Josef Prusa [Internet]. Prusa3D. 2019 [cited 2021 July 29]. Available from: https://www.prusa3d.com/.
- ↑ Ultimaker. Ultimaker 3D printers: Reliable and easy to use [Internet]. ultimaker.com. [cited 2021 July 29]. Available from: https://ultimaker.com/3d-printers.
- ↑ Fargo Additive Manufacturing Equipment 3D, LLC. 3D printers, parts, and filament [Internet]. LulzBot. [cited 2021 July 29]. Available from: https://www.lulzbot.com/.
- ↑ Ultimaker. Ultimaker PLA. [Internet]. Utrecht (Netherlands): Ultimaker BV; 2021 [cited 2021 Aug 20]. Available from: https://ultimaker.com/materials/pla.
- ↑ Werz SM, Zeichner SJ, Berg BI, Zeilhofer HF, Thieringer F. 3D printed surgical simulation models as educational tool by maxillofacial surgeons. Eur J Dent Educ. 2018;22(3):e500–5. https://doi.org/10.1111/eje.12332.
- ↑ Legocki AT, Duffy-Peter A, Scott AR. Benefits and Limitations of Entry-Level 3-Dimensional Printing of Maxillofacial Skeletal Models. JAMA Otolaryngol Head Neck Surg. 2017;143(4):389–394. doi:10.1001/jamaoto.2016.3673.
- ↑ Internet for All: A Framework for Accelerating Internet Access and Adoption (White Paper). World Economic Forum, 12 May 2016. URL: https://www.weforum.org/reports/internet-for all-a-framework-for-accelerating-internet-access-and-adoption.
- ↑ International Telecommunication Union (ITU), 2015, ICT Facts & Figures URL: https://www.itu.int/en/ITU-D/Statistics/Documents/facts/ICTFactsFigures2015.pdf.
- ↑ The Mobile Economy: Sub-Saharan Africa 2020. GSM Association. URL: https://www.gsma.com/mobileeconomy/wp-content/uploads/2020/09/GSMA_MobileEconomy2020_SSA_Eng.pdf.
- ↑ Mahler DG, Yonzan N, Lakner C, Aguilar RAC, Wu H. Updated Estimates of the Impact of Covid-19 on Global Poverty: turning the corner on the pandemic in 2021? [Internet]. World Bank Blogs. 2021 [cited 2021 Dec 10]. Available from: https://blogs.worldbank.org/opendata/updated-estimates-impact-covid-19-global-poverty-turning-corner-pandemic-2021.
- ↑ 34.0 34.1 AIGE Limited. 3D printers. [Internet]. 3D Printers | AIGE Limited. [cited 2021 Nov 3]. Available from: https://www.aige.info/3d-printers.
- ↑ 35.0 35.1 35.2 Riders for Health. Medical supply chain logistics. [Internet]. Olney (MD): Riders for Health II; 2021 [cited 2021 Aug 17]. Available from: https://www.riders.org/how-we-work/services/distribution-of-pharmaceuticals-and-medical-supplies/.
- ↑ WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition. Geneva: World Health Organization; 2021. Licence: CC-BY-NC-SA-3.0 IGO. The World Health Organization (WHO) is not responsible for the content or accuracy of any translation. The original English edition shall be the binding and authentic edition.
- ↑ Ronsmans C, Graham WJ, Lancet Maternal Survival Series steering group. Maternal mortality: who, when, where, and why. Lancet. 2006;368(9542):1189–200. doi: 10.1016/S0140-6736(06)69380-X.
- ↑ Ahmed S, Genadry R, Stanton C, Lalonde AB. Dead women walking: neglected millions with obstetric fistula. Int J Gynaecol Obstet. 2007 Nov;99 Suppl 1:S1–3.
