This literature review is meant to provide an understanding of the usefulness and properties of perovskites as a material for 3D printing solar cells.
This lit review needs to be reorganized into categories related to each layer and the materials each layer could be printed with.Furthermore, a section on printing powdered materials in a solvent should be added.
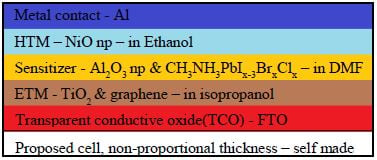
Current Design[edit | edit source]
- Metal contact - Aluminum Metal
- Hole Transporting Material - NiO np
- Sensitier - CH3NH3PbIx-3BrxClx
- Mesoscopic Layer - Al2O3 np
Al2O3 gamma np - Reasoning for gamma over alpha: highly porous, potentially catalytic features - need paper citation
- Electron Transporting Material - TiO2 & graphene flakes
- Transparent Conducting Oxide - Florine doped Tin-Oxide
Direction[edit | edit source]
Reusing lead "Recycling lead acid batteries into perovskite solar cells."
Used for syringe design Open-source syringe pump
3D printer used and modified Mendel (iteration 2)
Used for Raspberry Pi set up on prusa for franklin How to install FLIR Lepton Thermal Camera and applications on Raspberry Pi
Modified parts[edit | edit source]
- Upload images and then upload to github.
Raspberry Pi Case http://www.thingiverse.com/thing:30572 SlideHolder http://www.thingiverse.com/thing:232822
Basic Knowledge[edit | edit source]
Perovskite
Material Properties
Solar Energy
3D printing
MSDS Sheets[edit | edit source]
HTM Layer
Nickel(II) acetate tetrahydrate
ETM Layer Titanium(IV) Isopropoxide
p-type material layer(NiO)[edit | edit source]
p-type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells[edit | edit source]
Link: http://www.nature.com/articles/srep04756
Kuo-Chin Wang J-YJ. "p-type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells" Scientific reports. 2014;4:4756.
- This paper was the initial reasoning for using NiO as a p-type metal oxide HTM material
Fabrication of NiOx solution The precursor for NiOx film coating was prepared with 0.5 M nickel formate dihydrate (Alfa Aesar) in ethylene glycol solution containing 1 M ethylenediamine (Aldrich) and filtered with 0.45 μm nylon filters41.
Fabrication of NiOnc paste The mesoporous NiO solution used for spin coating was prepared by diluting slurry NiO with anhydrous ethanol in a ratio of 1:7. Slurry NiO was prepared by mixing 3 g of NiO nanopowder (Inframat) in 80 ml ethanol and subsequently adding with 15 g of 10 wt% ethyl cellulose (in EtOH) and 10 g of terpineol. The solution was stirred and dispersed with ultrasonic horn and concentrated with rotary evaporator for ethanol removal until 23 mbar
- Goal is to encompass the perovskite inside metal oxides to massively increase stability of the cell.
Properties of Plasmon-Induced Photoelectric Conversion on a TiO2/NiO p–n Junction with Au Nanoparticles[edit | edit source]
Link: Nakamura, K., Oshikiri, T., Ueno, K., Wang, Y., Kamata, Y., Kotake, Y., Misawa, H., 2016. Properties of Plasmon-Induced Photoelectric Conversion on a TiO2/NiO p–n Junction with Au Nanoparticles. J. Phys. Chem. Lett. 7, 1004–1009. doi:10.1021/acs.jpclett.6b00291[1]
Review of low cost synthesis of perovskite solar cells
Free source: [3]
Abstract (Just accepted article)
We have successfully fabricated all-solid-state plasmonic photoelectric conversion devices composed of titanium dioxide (TiO2)/nickel oxide (NiO) p-n junctions with gold nanoparticles (Au-NPs) as prototype devices for a plasmonic solar cell. The characteristics of the crystal structures and the photoelectric properties of the all-solid-state devices were demonstrated. We observed that the crystalline structure of the NiO thin film and the interfacial structure of TiO2/Au-NPs/NiO changed significantly during an annealing treatment. Furthermore, the photoelectric conversion devices exhibited plasmon-induced photocurrent generation in the visible-wavelength region. The photocurrent may result from plasmon-induced charge separation. The photoelectric conversion properties via plasmon-induced charge separation were strongly correlated with the morphology of the TiO2/Au-NPs/NiO interface. The long-term stability of the plasmonic photoelectric conversion device was found to be very high because a stable photocurrent was observed even after irradiation for 3 days.
- Good info on the use of NiO and TiO2
Solution deposited NiO thin-films as hole transport layers in organic photovoltaics[edit | edit source]
Abstract
Organic solar cells require suitable anode surface modifiers in order to selectively collect positive charge carriers and improve device performance. We employ a nickel metal organic ink precursor to fabricate NiO hole transport layers on indium tin oxide anodes. This solution deposited NiO annealed at 250 C and plasma treated, achieves similar OPV device results reported with NiO films from PLD as well as PEDOT:PSS. We demonstrate a tunable work function by post-processing the NiO with an O2-plasma surface treatment of varied power and time. We find that plasma treatment is necessary for optimal device performance. Optimal devices utilizing a solution deposited NiO hole transport layer show lower series resistance and increased fill factor when compared to solar cells with PEDOT:PSS.
- Good for scientific reasoning to not use organics for HTM
- Solid background on NiO
- Utilizies a NiO ink patented by the NREL, however it requires plasma treatment
Solution-Processed Nickel Oxide Hole Transport Layers in HighEffi ciency Polymer Photovoltaic Cells[edit | edit source]
Abstract
The detailed characterization of solution-derived nickel (II) oxide (NiO) holetransporting layer (HTL) fi lms and their application in high effi ciency organic photovoltaic (OPV) cells is reported. The NiO precursor solution is examined in situ to determine the chemical species present. Coordination complexes of monoethanolamine (MEA) with Ni in ethanol thermally decompose to form non-stoichiometric NiO. Specifi cally, the [Ni(MEA) 2(OAc)] + ion is found to be the most prevalent species in the precursor solution. The defect-induced Ni 3 + ion, which is present in non-stoichiometric NiO and signifi es the p-type conduction of NiO, as well as the dipolar nickel oxyhydroxide (NiOOH) species are confi rmed using X-ray photoelectron spectroscopy. Bulk heterojunction (BHJ) solar cells with a polymer/fullerene photoactive layer blend composed of poly-dithienogermole-thienopyrrolodione (pDTG-TPD) and [6,6]-phenyl-C71-butyric acid methyl ester (PC 71 BM) are fabricated using these solution-processed NiO fi lms. The resulting devices show an average power conversion effi ciency (PCE) of 7.8%, which is a 15% improvement over devices utilizing a poly(3,4 ethylenedioxythiophene):poly(styrenesulf onate)(PEDOT:PSS) HTL. The enhancement is due to the optical resonance in the solar cell and the hydrophobicity of NiO, which promotes a more homogeneous donor/acceptor morphology in the active layer at the NiO/BHJ interface. Finally, devices incorporating NiO as a HTL are more stable in air than devices using PEDOT:PSS.
- Signifies that an in order to deposit an ultra-thin layer of NiO, a solution stabilizer (amine) is needed, in this case monoethanolamine.
- 5nm thickness was achieved allowing for 95% light transmittance.
NiO Precursor Solution: Nickel acetate tetrahydrate(Ni(CH 3COO) 2· 4H 2 O) (Acros Organics) was dissolved in ethanol with monoethanolamine (NH 2CH 2CH 2 OH) (Sigma-Aldrich) (0.1 mol L − 1). The mole ratio of Ni 2 +: MEA was maintained at 1:1 in solution. Dissolution took place while stirring in a sealed glass vial under air at 70 ° C for 4 h. The solution appeared homogeneous and deep green after approximately 40 min.
Ultrafast Dynamics of Hole Injection and Recombination in Organometal Halide Perovskite Using Nickel Oxide as p-Type Contact Electrode[edit | edit source]
Abstract
There is a mounting effort to use nickel oxide (NiO) as p-type selective electrode for organometal halide perovskite-based solar cells. Recently, an overall power conversion efficiency using this hole acceptor has reached 18%. However, ultrafast spectroscopic investigations on the mechanism of charge injection as well as recombination dynamics have yet to be studied and understood. Using time-resolved terahertz spectroscopy, we show that hole transfer is complete on the subpicosecond time scale, driven by the favorable band alignment between the valence bands of perovskite and NiO nanoparticles (NiO(np)). Recombination time between holes injected into NiO(np) and mobile electrons in the perovskite material is shown to be hundreds of picoseconds to a few nanoseconds. Because of the low conductivity of NiO(np), holes are pinned at the interface, and it is electrons that determine the recombination rate. This recombination competes with charge collection and therefore must be minimized. Doping NiO to promote higher mobility of holes is desirable in order to prevent back recombination.
- Organometal lead halide perovskite based materials are attractive research topics because of long charge diffusion lengths, broad absorption ranges from visible to infrared, high absorption coefficints, low exciton binding energy, and a direct optical bandgap around 1.5eV.
- NiO shows to have a hole injection similar in time scale to that of electron injection into TiO2.
- Recombination occurs sometime between several hundred picoseconds to a few nanoseconds due to the pinning holes at the interface between NiO and perovskite material.
- Emphasises a need of doping NiO in order into increase the lifetime of electrons and holes.
- This paper has solid photoconductivity kinetics data.
Effects of Cu doping on nickel oxide thin film prepared by sol–gel solution process[edit | edit source]
Abstract
We prepared nickel oxide (NiO) thin films with p-type Cu dopants (5 at%) using a sol–gel solution process and investigated their structural, optical, and electrical characteristics by X-ray diffraction (XRD), atomic force microscopy (AFM), opticaltransmittance and current–voltage (I–V) characteristics. The crystallinity of the NiO films improved with the addition of Cu dopants, and the grain size increased from 38 nm (nondoped) to 50 nm (Cu-doped). The transmission of the Cu-doped NiO film decreased slightly in the visible wavelength region, and the absorption edge of the film red-shifted with the addition of the Cu dopant. Therefore, the width of the optical band gap of the Cu-doped NiO film decreased as compared to that of the non-doped NiO film. The resistivity of the Cu-doped NiO film was 23 m, which was significantly less than that of the non-doped NiO film (320 m). Thus, the case of Cu dopants on NiO films could be a plausible method for controlling the properties of the films.
- Useful if processing to dope NiO with Cu.
- Chemicals used:
- Nickel acetate tetrahydrate(Ni(COOCH3)2·4H2O, 0.3 M)
- Copper acetate monohydrate (Cu(CH3COO)2·H2O, 5 at%)
- Both dissolved in: 2-methoxyethanol (2ME), and hydrochloric acid (HCl)
- The solution was stirred at 60 ◦C for 1 h and then aged for 24 h at room temperature. Annealed at 550C for 1 hour
[http://ieeexplore.ieee.org/stamp/stamp.jsp?tp[edit | edit source]
&arnumber=7355717 Simulation of perovskite solar cells with inorganic hole transporting materials]===
Abstract
Device modeling organolead halide perovskite solar cells with planar architecture based on inorganic hole transporting materials (HTMs) were performed. A thorough understanding of the role of the inorganic HTMs and the effect of band offset between HTM/absorber layers is indispensable for further improvement in power conversion efficiency (peE). Here, we investigated the effect of band offset between inorganic HTM/absorber layers. The solar cell simulation program adopted in this work is named wxAMPS, an updated version of the AMPS tool (Analysis of Microelectronic and Photonic Structure).
- Useful for direction of copper doped NIO layer or Cu2O layer
- Regard for future work on NiO
Introducing Cu2O Thin Films as a Hole-Transport Layer in Efficient Planar Perovskite Solar Cell Structures[edit | edit source]
Abstract
In this work, we introduce Cu2O thin films as a hole-transport layer in planar perovskite solar cells. Here, a Cu2O layer was formed through successive ionic layer adsorption and reaction (SILAR) method. With methylammonium lead triiodide (MAPbI3) we form a direct structure (p− i−n), where the perovskite layer is sandwiched between a layer of p-type Cu2O and another layer of n-type PCBM (phenylC61-butyric acid methyl ester), which acted as hole- and electron-transport materials, respectively. We locate band edges of the materials with respect to their Fermi energy by recording scanning tunneling spectroscopy that has correspondence to their density of states (DOS). We observe that the energy levels of the materials form type II band alignments at each of the two interfaces (p−i and i−n) for charge separation and uninterrupted carrier transport upon illumination. Such a band alignment enabled charge transfer from MAPbI3 as evidenced from quenching of its photoluminescence emission when the perovskite was in contact with either the hole- or the electron-transport layer. With the direct p−i−n structure having appropriate energy levels for carrier separation, the planar perovskite solar cell (Cu2O/MAPbI3/PCBM) yielded an energy conversion efficiency (η) of 8.23% under 1 sun illumination.
- NiO alternative
High-Performance and Environmentally Stable Planar Heterojunction Perovskite Solar Cells Based on a Solution-Processed Copper-Doped Nickel Oxide Hole-Transporting Layer[edit | edit source]
- For use with Cu in NiO
Sol–gel deposited nickel oxide films for electrochromic applications[edit | edit source]
Abstract
The electrochromic (EC) behavior, the microstructure, and the morphology of sol–gel deposited nickel oxide (NiOx) coatings were investigated. The films were produced by spin and dip-coating techniques on indium tin oxide (ITO)/glass and Corning glass (2947) substrates. The coating solutions were prepared by reacting nickel(II) 2-ethylhexanoate as the precursor, and isopropanol as the solvent. NiOx was heat treated at 350 °C for 1 h. The surface morphology, crystal structure, and EC characteristics of the coatings were investigated by scanning electron microscopy (SEM), electron dispersive spectroscopy (EDS), atomic force spectroscopy (AFM), X-ray diffractometry (XRD), and cyclic voltammetry (CV). SEM and AFM images revealed that the surface morphology and surface characteristics of the spin- and dip-coated films on both types of substrate were different. XRD spectra revealed that both films were amorphous, either on ITO or Corning glass substrates. CV showed a reversible electrochemical insertion or extraction of the K+ ions, cycled in 1 M KOH electrolyte, in both type of film. The crystal structure of the cycled films was found to be XRD amorphous. Spectroelectrochemistry demonstrated that dip-coated films were more stable up to 1000 coloration–bleaching cycles, whereas spin-coated films gradually degraded after 500 cycles.
- useful for comparing dip technique to the spin coating technique. Good images.
Structural and Electrical Functionality of NiO Interfacial Films in Bulk Heterojunction Organic Solar Cells[edit | edit source]
Abstract
The functionality of NiO interfacial layers in enhancing bulk heterojunction (BHJ) organic photovoltaic (OPV) cell performance is investigated by integrated characterization of the electrical properties, microstructure, electronic structure, and optical properties of thin NiO films grown on glass/ITO electrodes. These NiO layers are found to be advantageous in BHJ OPV applications due to favorable energy band levels, interface passivation, p-type character, crystallinity, smooth surfaces, and optical transparency. The NiO overlayers are fabricated via pulsed-laser deposition and found to have a work function of ∼5.3 eV. They are investigated by both topographic and conductive atomic force microscopy and shown to passivate interfacial charge traps. The films also have an average optical transparency of >80% in the visible range, crucial for efficient OPV function, and have a near-stoichiometric Ni:O surface composition. By grazing-incidence X-ray diffraction, the NiO thin films are shown to grow preferentially in the (111) direction and to have the fcc NaCl crystal structure. Diodes of p�n structure and first-principles electronic structure calculations indicate that the NiO interlayer is preferentially conductive to holes, with a lower hole charge carrier effective mass versus that of electrons. Finally, the implications of these attributes in advancing efficiencies for state-of-the-art OPV systems—in particular, improving the open circuit voltage (VOC)—are
- Critical Paper
p-Type semiconducting nickel oxide as an efficiency-enhancing anode interfacial layer in polymer bulk-heterojunction solar cells[edit | edit source]
Abstract
To minimize interfacial power losses, thin (5–80 nm) layers of NiO, a p-type oxide semiconductor, are inserted between the active organic layer, poly(3-hexylthiophene) (P3HT) + [6,6]-phenyl-C61 butyric acid methyl ester (PCBM), and the ITO (tin-doped indium oxide) anode of bulk-heterojunction ITO/P3HT:PCBM/LiF/Al solar cells. The interfacial NiO layer is deposited by pulsed laser deposition directly onto cleaned ITO, and the active layer is subsequently deposited by spin-coating. Insertion of the NiO layer affords cell power conversion efficiencies as high as 5.2% and enhances the fill factor to 69% and the open-circuit voltage (V oc) to 638 mV versus an ITO/P3HT:PCBM/LiF/Al control device. The value of such hole-transporting/electron-blocking interfacial layers is clearly demonstrated and should be applicable to other organic photovoltaics. discussed.
- important for demonstrating NiO effect on a cell.
- 5-10 nm thickness of layer is shown to be ideal with significant PCE drop if the layer thickness is increased to even just 30nm.
Transparent conducting p-type NiO thin films prepared by magnetron sputtering[edit | edit source]
Abstract
Transparent and conductive thin films consisting of p-type nickel oxide (NiO) semiconductors were prepared by r.f. magnetron sputtering. A resistivity of 1.4 × 10−1 ohms cm and a hole concentration of 1.3 × 1019 cm−3 were obtained for non-intentionally doped NiO films prepared at a substrate temperature of 200°C in a pure oxygen sputtering gas. An average transmittance of about 40% in the visible range was obtained for a 110 nm thick NiO film. A semitransparent thin film pin diode consisting of p-NiO/i-NiO/i-ZnO/n-ZnO layer having a voltage-current rectification characteristic and an average transmittance above 20% in the visible range was fabricated on a glass substrate.
TiO2/Graphene or ZnO (n-type Layer)[edit | edit source]
TiO2
Low-Temperature Processed Electron Collection Layers of Graphene/TiO2 Nanocomposites in Thin Film Perovskite Solar Cells[edit | edit source]
Low-Temperature Processed Electron Collection Layers of Graphene/TiO2 Nanocomposites in Thin Film Perovskite Solar Cells Supporting Information[edit | edit source]
Measurement of Multicomponent Solubility Parameters for Graphene Facilitates Solvent Discovery[edit | edit source]
High-yield production of graphene by liquid-phase exfoliation of graphite[edit | edit source]
High-yield production of graphene by liquid-phase exfoliation of graphite Supporting Information[edit | edit source]
Solution processable titanium dioxide precursor and nanoparticulated ink: application in Dye Sensitized Solar Cells[edit | edit source]
Solution processable titanium dioxide precursor and nanoparticulated ink: Application in Dye Sensitized Solar Cells[edit | edit source]
Preparation of TiO2 Sol Using TiCl4 as a Precursor[edit | edit source]
Hydrolysis preparation of the compact TiO2 layer using metastable TiCl4 isopropanol/water solution for inorganic–organic hybrid heterojunction perovskite solar cells[edit | edit source]
Approach[edit | edit source]
Speciation in diethanolamine-moderated TiO2 precursor sols and their use in film formation[edit | edit source]
Photo-induced monomer/dimer kinetics in methylene blue degradation over doped and phase controlled nano-TiO2 films[edit | edit source]
Transparent thin films of Cu–TiO2 with visible light photocatalytic activity[edit | edit source]
9 mL of titanium(IV) isopropoxide was added to 120 mL isopropanol. Subsequently 0.3 mL 2 M HCl was added drop by drop.
Effects of solvent on properties of sol—gel-derived TiO2 coating films[edit | edit source]
ZnO
Origin of the Thermal Instability in CH3NH3PbI3 Thin Films Deposited on ZnO[edit | edit source]
Electroluminescence from light-emitting polymer/ZnO nanoparticle heterojunctions at sub-bandgap voltages[edit | edit source]
Perovskite Layer - active layer[edit | edit source]
Ultrasmooth organic–inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells[edit | edit source]
Abstract
To date, there have been a plethora of reports on different means to fabricate organic–inorganic metal halide perovskite thin films; however, the inorganic starting materials have been limited to halide-based anions. Here we study the role of the anions in the perovskite solution and their influence upon perovskite crystal growth, film formation and device performance. We find that by using a non-halide lead source (lead acetate) instead of lead chloride or iodide, the perovskite crystal growth is much faster, which allows us to obtain ultrasmooth and almost pinhole-free perovskite films by a simple one-step solution coating with only a few minutes annealing. This synthesis leads to improved device performance in planar heterojunction architectures and answers a critical question as to the role of the anion and excess organic component during crystallization. Our work paves the way to tune the crystal growth kinetics by simple chemistry.
- Critical paper
- Tio2 process to be repeated if low temp graphene layer process does not work: "A hole-blocking layer of compact TiO2 was deposited by spin-coating a mildly acidic solution of titanium isopropoxide in ethanol, and annealed at 500 °C for 30 min. Spin-coating was carried out at 2,000 r.p.m. for 60
3D printing papers[edit | edit source]
Toward Large Scale Roll-to-Roll Production of Fully Printed Perovskite Solar Cells[2][2][edit | edit source]
Abstract
Solar cell technology has been developed to harvest solar energy more efficiently as well as more economically. Third-generation solar cells, including chemical-compound solar cells (CIGS, CdTe), dye-sensitized solar cells (DSSCs), and organic photovoltaics (OPVs), have been intensively studied during the past decade for their potential low production costs. [ 1–6 ] Recently, organic-inorganic hybrid perovskite solar cells have emerged as the most promising of the third-generation solar cells with an increased record of efficiency that has risen to 19.3% [ 7 ] from 3.8% [ 8 ] in last 4 years. In addition to record efficiencies published in the literature, a certified record efficiency of over 20% [ 9 ] has been reported very recently, albeit with no details disclosed. This record efficiency is already comparable to that of silicon solar cells. [ 9 ] The next challenge in the field will be translating the lab-scale process to a large-scale production process, which will preferably include a cost-competitive roll-to-roll printing process.
- This is a very useful paper as it proves a methodology to create a soloar cell of PCE: 11.94%.
- ITO/ZnO/MAPbI3/P3HT/Ag is the architecture used.
- ITO was sonicated
- ZnO np were coated and annealed at 120C for 10 minutes
- PbI2 in N, N -dimethylformamide was stirred for 1h at 70C then coated and dried with N2 and transferred to an enclosed space.
- CH3NH3I was synthesized and coated onto the PbI2 layer with bed temp at 70C.
- P3HT was immediately deposited to prevent moisture from damaging the perovskite layer.
- Ag was deposited via ion-deposition
- Entire process was carried out at ambient temp and humidity.
- Created a 10mm^2 section for testing
- This process can easily be adapted from a slot-die to a syringe pump process.
- ZnO is an alternative to TiO2, may be beneficial for reasons such as deposition temperature/ precursor solution(verify).
- The intrinsic layer is a good layer in this set up, they went through a lot of testing for printing optimization of the PbI2, worthy of modeling.
- I would use the NiO instead of P3Ht due to chemicals used, and it appears NiO should be easy to deposit.
- Overall, based on this design the process could take a few hours to do in a lab, but could easily be scaled up for industry.