Discussion II[edit | edit source]
The first question to be answered, as far as the microbial treatment of lignocellulose is concerned, is: What is the aim of the treatment? It is possible to produce biomass, biogas, or ethanol. The emphasis at this conference seemed to be on biomass production, but it must be admitted that some of the methods proposed do not seem very practical. One chemical treatment before inoculation with a lignin-degrading organism was criticized as using an unnecessarily high concentration of sodium hydroxide, a statement that was disputed but without agreement being reached.
Fermentation in submerged cultures was not favoured for use under rural conditions, and a recommendation was made that surface culture, the so called "solid substrate" technology, should receive more attention. It was suggested that this method should be tried under field conditions. There was also a comment that time is being lost in transferring technology to the villages because of the insistence of research workers on perfecting every factor in the laboratory before taking a process into the field. Much of this work may have little relevance to rural situations where it would not be possible to apply the same constraints as in the laboratory. It was also suggested that, in selecting organisms, it would be advisable first to find out from existing published work what is known of their pathogenic or toxic characteristics.
The chemical treatment of straw offers various possibilities. Sodium hydroxide is very effective in increasing the digestibility of straw, and considerable work has been done on its use. However, its cost, the energy needed to make it, and its polluting effect on the environment all render it unsuitable in the long term for straw treatment in developing countries. Initial results from the use of urea are promising, those from calcium hydroxide less so, but a combination of the two would be worth investigating. It was again emphasized that the reagents and processes that might be suitable in industrialized, temperate-zone countries are not necessarily the best in rural areas of the tropical and sub-tropical countries.
Mini-fermentation technology to produce single-cell protein from molasses[edit | edit source]
Ir Ign. Suharto
National Institute for Chemistry, Indonesian Institute of Sciences, Bandung, Indonesia
Ir S. Redyowati B.
Faculty of Engineering, University of Gadjah Mada, Yogyakarta, Indonesia
Introduction[edit | edit source]
Indonesia is an archipelago of 13,367 islands with a total land area of about 1,907,950 square kilometres. A major problem is how to transport and distribute food commodities from one island to another.
The population of Indonesia in 1978 was about 140 million, and the net population growth rate is about 2.2 per cent per year. About 80 per cent of the population lives in rural areas and represents mostly lowincome groups. Some 70 per cent of the population lives in Java and Madura, which make up only 7 per cent of the total land area. Kalimantan, Sulawesi, and Sumatra make up 28, 10, and 25 per cent of the total land area, respectively, and are used as transmigration areas for people from Java. The intensity of agricultural land use in Java and Madura is about 0.07 hectares per person.
The third five-year development plan (Repelita lll) covers the period 1 April 1979 to 31 March 1984. According to the general pattern of long-term national development, as stated in Repelita lll, the priority of national development is still focused on the agricultural sector. A more intensive agricultural system in Indonesia will bring economic advantages, but it will also increase the problem of food processing, particularly within the rural areas and the new transmigration areas where people still tend to live in traditional ways.
Single-cell protein as a possibility for improving the protein supply[edit | edit source]
In Repelita lll, the protein supply and demand pattern is a problem because of the population growth rate. This increases the requirement for protein and better-quality foods in general. As a consequence, "better-quality foods" implies increased quantities of animal protein.
On the supply side, plant protein is not sufficient to supply total requirements, although the opening-up of new transmigration areas has been adding to food crop production. One way to improve the supply of animal protein for human consumption is to increase the production of animal feedstuffs.
Animal feed production at present is based on fish waste and plant protein sources, but because of their relatively high cost it is necessary to seek others. The new sources must (a) have a high nutritional value, (b) not be competitive with food for human consumption, (c) be economically feasible, and (d) be locally available.
It is possible to introduce single-cell protein (SCP) for animal feeding. Its production will use renewable resources and waste sources such as molasses. SCP can minimize the use of fish waste, soybean cake, peanut cake, etc. for animal feeds. This has been shown in poultry feeding trials.
Average feed consumption by one bird is 100 9 per day. In 1979, the total number of birds was 7,500, needing a total of 750,000 kg feed per day. About 10 per cent of poultry feed is from fish or soybean cake or rice bran, which means that 27,375,000 kg per year of fish, soybean cake, or rice bran could be saved if these materials were replaced by SCP, as shown in table 1. High-grade protein is supplied by some types of single cell micro-organisms.
Raw materials[edit | edit source]
TABLE 1. Quantities of Present Sources of Protein in Feeds for Various Animals That Could Be Saved by Partial Replacement with Single-Cell Protein
|
SCP in Compound Feed (kg/ton) |
Replaced Protein Source |
% of Total Feed Protein Contributed by SCP | ||
|
Source |
Quantuty (kg/ton) | |||
| Broilers | 100 | SBM | 182 | 36 |
| Laying hens | 80 | SBM | 145 | 38 |
| Turkeys | 50 | FM | 62 | 18 |
| Pigs | 100 | SBM | 182 | 50 |
| Veal calves | 50 | SMP | 114 | 17 |
| Trout | 250 | FM | 308 | 44 |
Source: Or. Dimmling, Unde GmbH, Dortmund, FRG.
a. SBM = soybean meal; FM = fish meal; SMP = skim-milk powder.
Agro-industrial wastes, particularly molasses and sugar syrup, are available in Indonesia. The current status of sugar production in Indonesian factories is increasing not only in quality but also in quantity. In the past ten years, 56 sugar cane factories have processed 12 million tons of cane per year into 1.4 million tons of cane sugar and 480,000 tons of molasses. In Repelita 111, the government has launched a mini-technology for sugar cane factories that are spread throughout such islands as Sumatra, Kalimantan, and Sulawesi.
Three mini-technologies for sugar cane factories have already been set up in Aceh, West Sumatra, and Kalimantan. The capacity of each factory is 2,000 tons per year. The target of this plan is to establish about 200 mini sugar factories. The private sector plans to erect seven mini sugar factories outside Java. One of the aims of the mini sugar factories is to create a model in order to encourage the private sector to erect more factories of a similar kind.
It is clear that the higher the total cane sugar production, the higher the total availability of molasses. The production of SCP from molasses by using mini-fermentation technology is relevant to rural development and particularly to increasing per capita income. Some considerations in the selection of molasses as a raw material are (i) its year-round availability, contributing to the development of mediumand small scale industries throughout Indonesia, (ii) its potential for helping maintain the efforts of lowincome farmers and decreasing unemployment in rural communities, and (iii) the encouragement these factors may be expected to give to an increase in the spontaneous and regular flow of transmigrants from Java to other islands.
The objectives of this project are:
- to study the properties of micro-organisms that are not pathogenic or toxic and have high protein and carbohydrate contents, a rapid growth rate, etc.;
- to study on the laboratory research scale optimum conditions of fermentation, product recovery, safety, improvement of products, etc.;
- to study kinetic analysis of SCP fermentation;
- to study and evaluate the pilot plant for SCP production from molasses;
- to scale up SCP production from the pilot-plant to the commercial scale;
- to carry out field trials of this SCP with broilers, layers, pigs, cattle, fish, etc.
Justification of the Project
Feed is a relatively high-cost item in the production of meat, fresh milk, eggs, and broilers. One way to solve this problem is to develop and implement the use of SCP for animal feeding. SCP can replace some of the usual protein sources in feedstuffs; soybean meal, fish meal, or skim-milk powder will be replaced by SCP with an equivalent amount of protein.
SCPs have some advantages, such as that yeasts can easily be controlled genetically and their protein content is higher than that in conventional feedstuffs. A most important characteristic of these SCPs is their high protein content, ranging from 40 to 80 per cent of their dry weight on a crude protein basis.
The process, which has been in use for several years, is basically an aerobic fermentation, followed by recovery of the cells. The stoichiometry of SCP processes is:
Carbohydrate/yeast
1.68 CH2O + 0.19 NH3 + 0.68 O2 - 10 {CH17O0,5N0,19 ash} cells
+ 0.17 CO2 +1.14 H2O + 80,000 calories
Proposed work programme[edit | edit source]
The proposed work programme consists of the following.
1. Laboratory research on SCP from molasses to provide a more quantitative basis for future requirements:
- chemical analysis of molasses and products,
- selection of strains of micro-organisms,
- optimum conditions of the fermentation process,
- kinetic analysis of the fermentation product,
- oxygen transfer and uptake by micro-organisms in aerobic fermentation,
- media storage, cell separation, cell drying, and extraction of valuable components from SCP.
2. Establishment of a pilot or prototype plant, provided with all essential production elements including quality control, to facilitate:
- training of scientific manpower,
- scaling-up to commercial plant,
- field trials of SCP products for birds, fish, pigs, cattle, etc.
Approach and methodology[edit | edit source]
In preliminary research activities on the production of SCP, the scale of operation should be considered first, as this will be influenced by the capacity of the pilot plant and commercial scale in the future. The end-product of fermentation technology will be mini-fermentation technology, in terms of simple prodcedure, simple equipment, and low cost.
The capacity of commercial scale production is planned to be about 1,000 tons per year, using molasses as a raw material substrate. BY comparison, mini sugar factories in Indonesia were designed for a capacity of 2,000 tons of sugar per year. According to this information, the following is the sequence of capacity at each stage.
- The capacity of the commercial plant will be 1,000 tons of SCP per year, 3.3 tons or (3,300 kg) per day.
- The capacity of the pilot plant will be 100 tons of SCP per year, or 334 kg per day.
- The capacity of laboratory activities related to the scaling-up process will be 34 kg of SCP per day. This is possible by using six fermentors (4- to 8-litre capacity each).
Laboratory Research on SCP from Molasses
In preliminary research on bioconversion of molasses to SCP, some aspects of the necessary conditions for SCP production from molasses must be studied (pH range, temperature, mean molasses content, reproduction time, specific growth rate, cell density, productivity, yield, oxygen uptake, kinetic analysis, heat of fermentation, nutrient solution, essential amino acid content, energy and utilities required, selection of the best strains of micro-organisms, etc.). All of the variables must be correlated with the SCP product so that the data obtained can be used to set up the pilot plant.
On the laboratory scale, 2 per cent molasses is used as a substrate with a nutrient solution added. This substrate is then fermented with Fleischmann's active dry yeast in a special fermentor with a capacity of about 4 to 8 litres; its working capacity is 4 litres. The inoculum used is 20 per cent substrate and the temperature is 30° ± 0.5°C. Other parameters will be adjusted from a control panel. Fermentation time is ten hours.
The number of cells per litre and the oxygen absorption rate during fermentation can be calculated. The specific growth rate can be calculated using this equation:
X = XEkt where
X = total cells at t hour, k = specific growth rate
X = total cells at t
t = fermentation time
At an agitation rate of 325 rpm, the total number of cells will increase exponentially and will attain equilibrium after eight hours of fermentation. At the higher rate of 490 rpm, the total number of cells will attain equilibrium after seven hours of fermentation. This might be the result of the autocatalysis of cells. Further laboratory development will take place after the joint proposal on feed from agricultural and agro-industrial wastes has been approved.
Estimated cost of the programme[edit | edit source]
| Research and development will require a budget for: | |
|
- equipment (six fermentors with 4- to 8-litre capacity, laminar flow cabinet, spray drier, extraction unit, digital analytical balance, centrifuge, paramagnetic oxygen analyser, digital pH meter, etc.) |
US$400,000 |
| - chemicals and supplies | 150,000 |
| - salaries | 150,000 |
| - miscellaneous | 200,000 |
| Total (for three years) | US$900,000 |
| The investment required for SCP production with a capacity of 100 tons per year, or 334 kg per day, will be: | |
| - quoted equipment | US$450,000 |
| - estimated equipment installation cost | 50,000 |
| - piping | 50,000 |
| - instrumentation with some automatic controls | 75,000 |
| - auxiliaries (e.g., electric and steam power) | 75,000 |
| - buildings | 200,000 |
| - maintenance | 40 000 |
| - utilities | 100,000 |
| - engineering and construction fees | 50,000 |
| - salaries | 120,000 |
| - operation costs | 120,000 |
| - contingency | 170,000 |
| Total (for three years) | US$1,500,000 |
Summary[edit | edit source]
The demand for and supply of protein are not in balance, nor is the supply adequate for Indonesia's total population of about 140 million (1978), especially when considering the net population growth rate of 2.2 per cent per year and the difficulties of transportation in the archipelago.
To improve the supply of animal protein for human consumption it is necessary to increase the production of animal feedstuffs. SCP from molasses can replace some of the usual protein sources in feedstuffs. The availability of molasses is at least 480,000 tons per year, and this is increasing because mini sugar factories are operating on the islands outside Java. These experiments are to study the optimum conditions of fermentation and to set up a pilot plant and field trials for SCP production. A pilot plant will be set up to process 100 tons per year, the duration of the project will be three years, and the cost of the programmes for research and development and a pilot plant will be US$900,000 and US$1,500,000, respectively. The plant will be located in Bandung.
Bibliography[edit | edit source]
Anggorodi, R. "Penghematan Bahan Makanan Berprotein Tinggi dengan Ragi Hidro Karbon dalam Ransum Ternak." Jakarta, Indonesia, 1979.
Coursey, D.G. Cassava as Food: Toxicity and Technology. Tropical Products Institute, London, 1973.
"Engineering of Unconventional Protein Production." Chem. Eng. Progress, Symposium Series, 93:65 (1969).
Indonesian Institute of Engineers. Food Policy in Indonesia. 1979.
John, C.K, "Recycling of Agro-industrial Wastes." Science and Technology Seminar, Kuala Lumpur, Malaysia, 1978.
MacLennan, D.G. "Single-Cell Protein." PACE, April 1974, pp. 13-17,
Proceedings of the Second Poultry Science and Industry Seminar. Ciawi, Bogor, Indonesia, 1979.
Rogers, P.L. "Single-Cell Protein from Agricultural and Industrial Waste." Unpublished paper, University of New South Wales, Sydney, Australia.
Vilbrant, F.C. Chemical Engineering: Plant Design. McGraw-Hill Book Company, London, 1959.
Current status and utilization of carbohydrate residues in Indonesia[edit | edit source]
Ir Ign. Suharto
National Institute for Chemistry, Indonesian Institute of Sciences, Bandung, Indonesia
Introduction[edit | edit source]
The third five-year development plan (Repelita lll, 1979-1984) aims to increase the prosperity of the Indonesian people and lay down a firm foundation for future development. It will be necessary to strike a balance between the agricultural and industrial sectors to ensure economic growth and a more equitable distribution of income between rural inhabitants and city dwellers. The tendency for the poor to become poorer and the rich to get richer is one that needs to be redressed in the interests not only of the people themselves but also of national stability.
The economic growth envisaged during Repelita lll is still based on the agricultural and agro-industrial sectors. About 80 per cent of the 140 million population of Indonesia lives in rural areas, and both rural and city dwellers depend on agriculture for their incomes. But economic growth alone is not the final solution: it must be accompanied by parallel social development. There are important differences in social, political, and cultural influences between rural and city areas, some of which are as follows:
|
Rural areas |
City areas |
|
- lack of science and technology |
advanced science and technology |
|
- lack of trained management |
no lack of trained management |
|
- adequate natural resources |
no natural resources |
|
- protein-calorie malnutrition (PCM) |
low incidence of PCM |
|
- plentiful land area |
no land area |
|
- adequate manpower |
adequate scientific manpower |
|
- little political power |
strong political power |
Commodity trading is mainly in the hands of city people. The rural inhabitants tend to preserve their traditional way of life and remain in the low-income group, many existing below the poverty line. One way of improving their standard of living is to convert their farm residues into more valuable materials for which there is a use and a demand. In effect, this means increasing the value of residues as animal feeds.
Java comprises only 7 per cent of the total land surface but supports 88 million 163 per cent) of the 140 million people in Indonesia, with a consequent pressure on the land available for agriculture. On the other hand, outside Java the population density is comparatively low and agricultural land is plentiful. It should therefore be feasible to increase production to satisfy the demand for both food for people and animal feed.
General objectives[edit | edit source]
Many Indonesian farmers have only small agricultural holdings. To increase their incomes they will have to make greater use of crop residues to increase the quantity and quality of animal production. Residues occur at all stages of production, storage, and marketing. These are often wasted or used inefficiently. In either case, their potential value as animal feed is not fully realized.
As well as the effect on farmers' incomes of locally produced animal feed material, an increase in domestic feed production is important in other respects. These are:
- a reduction in the amount of foreign exchange needed to import feed;
- the strategic advantage of greater self-sufficiency in animal feed production:
- the ability to build up buffer stocks of animal feed against emergency situations;
- the need to increase animal production by improving animal nutritional status.
However, to obtain these advantages other factors must be taken into account, such as the availability and continuity of supply of the various types of residues and the cost of converting them into better-quality feed materials.
In summary, therefore, the general objectives of projects for increasing the use of agricultural residues are to:
- increase meat, milk, and egg production as well as the animal population;
- improve the standard of living and prosperity of farmers and other low-income groups in rural areas;
- ensure a more equitable distribution of the national income and reduce the gap between rich and poor;
- create and encourage private sector participation in the animal feedstuffs industry,
Main agro-industrial by-products[edit | edit source]
Most agro-industrial by-products are derived from oil palm, tapioca, and sugar cane production. The oil palm industry produces about 270,000 tons of palm oil each year. The by-products include about 1 million tons of liquid effluents, 270,000 tons of empty bunches, 100,000 tons of pericarp fibre, 160,000 tons of shells, and 80,000 tons of palm kernel cake. The cake is used in formulated animal feeds. The palm oil sludge may have a potential use in animal feed.
Over 110 million tons of tapioca meal are produced annually, but only a small amount is available for animal feeding. The sugar cane industry produces 4 million tons of bagasse, 400,000 tons of press mud, and the same amount of molasses. The latter can be used as such in animal feeds or form the substrate for single-cell protein manufacture.
Other agro-industrial residues include rice bran, fodder yeast, coconut press cake, sago meal and waste, soy sauce, soybean curd, and dairy plant wastes. All of these have a use, or a potential for use, in animal feeds. However, more research is needed to evaluate the nutritional characteristics of these materials and their suitability for different types of livestock. Other factors that require investigation are any possible toxicological properties and the effects of processing and handling on bacterial and mycotoxin contamination.
The total feed requirement of livestock in Indonesia, according to Nell and Rollinson, is:
- forage, 108,507,000 tons,
- low-quality concentrates, 1,825,000 tons,
- high-quality concentrates, 206,000 tons.
The percentages of these required in Java are 52.6, 38.0, and 55.1, respectively.
Development strategy[edit | edit source]
As has been stated earlier, the main objective is to become, as nearly as possible, self-sufficient in animal feed production by making better use of the organic residues that are available.
Small-scale fermentation processes have been practiced for some years in Indonesia and are generally known to the farmers. When successful they may have advantages over chemical processes because they need little capital investment to establish and maintain them. For these reasons it may be better to place more emphasis on the development of biological rather than chemical treatment of residues.
The application of fermentation technology would be aimed at preserving feed from spoilage and increasing its nutritional value. This, in turn, would require research and training in biotechnology and in the nutritional and toxicological evaluation of these products.
Whereas this aspect would be the responsibility of institutes or university departments competent to undertake the work, the technology of producing the feed materials would have to be capable of application at the rural level.
Ideally, the whole system of the development of suitable technology, its transfer to rural areas, and the evaluation and application of the resulting feed materials, should be contained within the national policy for development. To achieve this, planning for the future will need to provide the following for the agricultural sector:
- the means to study a systems approach to feed and nutrition policies; this should include the definition and analysis of alternative strategies;
- facilities for training in feed production and animal nutrition;
- equipment for research in chemistry and microbiology, the study of rural development, farm management, and agricultural economics.
Conclusions[edit | edit source]
The third five-year plan (Repelita lll) is intended to increase the general prosperity of Indonesia and to ensure a more equitable distribution of the national income. A major factor in this will be development of the agricultural sector, particularly in the area of using organic residues for animal feeding more effectively.
Both biological and chemical processes will be developed for this purpose. The nutritional and, where necessary, the toxicological characteristics of products resulting from these processes must be evaluated.
The processes themselves must be capable of application in rural areas on either a community or individual farm scale.
These developments and the facilities needed to carry them out should be seen in the general context of national development in which a balance is required in technical, social, economic, and political influence between city and rural areas.
An interdisciplinary approach to the problems is necessary, as is co-operation with both national and international scientific bodies,
Bioconversion of carbohydrate residues in Thailand[edit | edit source]
Malee Sundhagul and Poonsook Atthasampunna Bangkok Microbiological Resources Centre (MIRCEN), Thailand Institute of Scientific and Technological Research, Bangkok, Thailand
Introduction[edit | edit source]
Thailand, like many other developing countries in South-East Asia, is an agricultural country and, as such, produces several hundred million tons of agricultural products annually. Of this vast amount of vegetables, fruits, fish, meat, and other raw materials used for various kinds of edible and agroindustrial products, some millions of tons are wasted each year. These waste problems become pollutants causing increasing environmental problems. In the ASEAN region alone, for instance, it has been estimated that 30 million tons of rice grain are produced each year. This is accompanied by 140 million tons of straw, 13 million tons of husks, and 2 million tons of bran.
Considering similarly large quantities of other commodities, such as cassava, maize, and sugar cane, tremendous resources of organic raw materials are potentially available for conversion into useful products - the common "F" products: food, fuel, fertilizer, and fibre. Among many means of converting these waste materials - mechanical, chemical, and biological - bioconversion (particularly microbiological) seems to be the most suitable for Thailand, and probably for other tropical developing countries as well.
The vast reservoir of genetic resources of micro-organisms is increasingly being tapped and harnessed for its potential for the detoxification of wastes, the purification of polluted waste effluents, the traditional fermentation of foods and feeds, the production of vitamins and vaccines, the microbial fixation of nitrogen, and the production of biogas as fuel from manure.
The present paper describes the status of bioconversion of organic residues, with particular emphasis on carbohydrate residues.
The present status of bioconversion of carbohydrate residues in Thailand[edit | edit source]
Sources of Carbohydrate Residues
For the purpose of this discussion, it is proposed to categorize carbohydrate residues into three types: cellulosic, starchy, and sugary. In practice, cellulosic residues are mainly lignocellulosic and sometimes include starchy and/or sugary components. The following are major waste materials under each of these categories with high potential for bioconversion:
- cellulosic: rice straw, rice husks, corn stalks, corn cobs, bagasse, cane filter cake (mud), kenaf (Hibiscus cannabinus) stalks, animal manure, pineapple peels;
- starchy: cassava meal (residue), cassava effluent;
- sugary: molasses, distillery slops, pulp waste liquor, coconut water (juice).
Current Traditional Practices
Industries in Thailand have increasingly been recovering residues and wastes from raw materials and plant waste material as by-products in an attempt to reduce pollution problems as well as to minimize production costs for major food items. Cassava residues from starch factories have been recovered for animal feed, and fermentation broth from monosodium glutamate (MSG) factories is used as flavouring for sauces. However, here we will discuss only those methods currently practiced at the rural level with potential application in other areas.
In Thailand, as in other agricultural countries, cellulose is the most abundant organic waste. Consisting chemically of long chains of glucose, it is easily hydrolysed by cellulase enzymes normally produced by bacteria in ruminants. Therefore, one of the most common uses of cellulosic wastes has been as animal feed.
Non-cellulosic carbohydrate residues, such as starch in cassava and sugar-containing residues (molasses), are generally used as feeds for non-ruminants, i.e., pigs and poultry. Broken rice rejected in the milling process, barley, maize, and cassava meal are commonly used as feeds in Thailand. The use of these residues may be considered a form of bioconversion in common practice throughout the world.
Where cellulosic materials have high lignin components, digestion by animals is minimal. In such cases, a small amount of fermentable carbohydrates such as corn meal or molasses is added to produce silage, thus ensuring rapid fermentation. Silage making normally serves as one of the effective means of conserving high-moisture products for animal feed. Corn (maize) silage is most common in Thailand, and cassava is being used increasingly.
Many crop residues are deficient in protein and minerals. It is therefore not uncommon for farmers to add products such as urea and minerals at the time of ensiling.
Fermentation of carbohydrate residues for human consumption is negligible in Thailand. Small amounts of fermentable carbohydrates are sometimes used as adjuncts to ensure rapid fermentation of other easily perishable protein food, such as pork (fermented sour pork), fish (fermented sweet fish, or pla chao), etc.
Biogas production in Thailand has not received adequate attention. Recently, some attempts have been made to promote this type of bioconversion.
Current Research and Development Activities
There has been increasing interest in the productive utilization of agricultural and agro-industrial residues throughout the world.
International. Recently, the US National Academy of Sciences convened a panel of experts from different parts of the world with the objective of producing a document on the current status of utilization of organic waste materials to produce foods, fuel, and fertilizer. In December 1979, UNEP convened a meeting of policy makers and administrators on the subject of waste utilization as a followup of the previous consultative meeting of experts on this subject.
Regional. An ASEAN regional co-operative project has recently been launched by the ASEAN Subcommittee on Protein aimed at better utilization of food crop wastes. The emphases are on converting food waste into acceptable food, followed by making them suitable for animal feed, and as potential alternative sources of energy.
National. Several research and development programmes and projects are being carried out by universities and research institutions in Thailand. Those specifically involving carbohydrate raw materials are as follows:
- anaerobic digestion (and biogas production) of distillery slops,
- microbial preservation of bagasse for pulp production,
- single-cell protein (SCP) production from pineapple waste,
- protein enrichment of cassava residue,
- microbial fertilizer (Rhizobium) from cane filter cake.
Application of bioconversion of carbohydrate residues[edit | edit source]
In applying knowledge of converting residues into useful by-products, several factors must be taken into consideration in order to ensure successful implementation. The four major factors are technical, economic, socio-cultural, and, finally, political - all, in most cases, interrelated.
Technological Considerations
Biological conversion or bioconversion of carbohydrate residues in Thailand mainly involves traditional fermentation processes that rely on natural inoculation of successive fermentation using part of a previous batch as the inoculum for the following batch. At a more sophisticated level of fermentation technology, pure inoculum is normally used. Fermentation technology has long been used to preserve easily perishable food materials and to produce a variety of foods and feeds.
To help strengthen the efforts to promote conservation, distribution (preservation), and environmentally sound management of available natural resources in Thailand and other South-East Asian countries, the Government of Thailand has established the Bangkok MIRCEN, which serves as a local centre for the conservation of microorganisms of economic and environmental significance, and for disseminating information relating to fermentation technology, particularly in the areas of food/feed fermentation and waste recycling.
At present, several technologies for converting carbohydrate residues into useful and value-added byproducts are known throughout the world and within the South-East Asian region. For village-scale technology, experience in Thailand reveals that those available now are adequate. The problem is how to create awareness among technologists and how to effectively transfer those technologies to the grassroots level for implementation. Information exchange, effective promotion, and support - particularly from the government - are considered most important.
Economic Considerations
In general, the adoption and implementation of bioconversion technology will depend largely upon the economic feasibility of integrating these practices into existing agricultural and agro-industrial residue management schemes. In most cases of rural agricultural and agro-industrial operations, collection of residues in a large enough quantity to be economical to process is a major problem. Transportation adds to raw material costs and leads to problems such as need for adequate storage facilities and deterioration in quality. In many instances, transportation facilities are not adequate.
Where recovery or treatment of residues is necessary largely for environmental reasons, potential economic benefit is rarely realized. In such cases, it is anticipated that at least operating costs can be recovered.
A survey was conducted in Thailand to identify socio-economic issues surrounding the practical feasibility of wide-scale promotion of biogas generation. It was found that most farmers were generally aware of the technology, but economics were the primary concern. They were found to be understandably conservative regarding additional capital expenditure. This attitude is also true among industrial entrepreneurs in the country, with the exception of those operating joint ventures with foreign counterparts.
Socio-cultural Considerations
The adaptation of new technologies to existing socio-cultural and economic systems has always been a complex process. The issues are more serious among villagers in rural areas with little or no education. In a survey to promote biogas generation from combined human and animal wastes, several people viewed the approach and proposed technology as unethical. Several others interviewed would not utilize biogas generated from pig manure, and many would not handle the manure.
Political Considerations
Political decisions and government measures are sometimes necessary in promoting the adoption and implementation of waste utilization schemes. This is particularly true in the area of waste treatment to solve pollution problems where economic benefits are not the primary objective.
Conclusions
Maximum and proper utilization of farm and agro-industrial wastes will inevitably result in better environmental management and additional income-generating sources, thus improving the quality of life for the rural poor. Improvement in agricultural productivity by using spent compost in the soil, thereby minimizing reliance on expensive inorganic fertilizers, will increase availability of foods and feeds, etc.
Support and encouragement by the policy makers, planners, educators, and implementors are necessary to help realize the above-mentioned goals.
In the coming decades, Thailand, as well as many other developing countries in the region with an abundant supply of agricultural raw materials, will rely significantly on beneficial microbes in their bioconversion efforts to meet the crises precipitated in food, fuel, environmental and other socioeconomic sectors.
Use of carbohydrate residues in Malaysia[edit | edit source]
R.l. Hutagalung
Department of Animal Sciences, University Pertanian Malaysia, Serdang, Selangor, Malaysia
Introduction[edit | edit source]
The dwindling food and feed reserves in the world have increased interest in the exploitation of carbohydrate residues that at present largely go to waste and are a pollution hazard. Within the past decade fresh impetus has been given to the serious study of these carbohydrate residues as substrates for the production of protein enriched foods or feeds through microbial fermentation. Part of this impetus has stemmed from wider recognition of malnutrition in the developing countries and efforts to combat it. At the same time, with the ever-increasing seriousness of the waste problems from the processing of food and natural carbohydrate sources, the production of microbial protein from these wastes and by-products could be a profitable way of overcoming this difficulty.
Carbohydrate residues are available in large quantities in many parts of South-East Asia. Some of these residues have been used as substrates to grow micro-organisms, and their nutritive value has been documented (1).
In Malaysia, as in many of her neighbouring countries, there are increasing needs for protein sources. Protein consumption has been reported to be about 45 g/day/person and to consist of not more than 17 g of animal protein. Efforts have been made to increase animal protein sources, such as meat from poultry and beef. However, with the high cost of imported concentrated feeds, especially of protein, meat production will eventually become economically unattractive.
Realizing these facts, considerable research has been conducted in Malaysia within the past ten years - and is currently being intensified - to maximize the use of various agro-industrial wastes, including those of carbohydrate residues, for useful animal feed and thus, indirectly, for food.
This paper presents the broad outline of the current work done in Malaysia on the use of carbohydrate residues, and the advances made toward the realization of finished products for large-scale application.
Carbohydrate residues available[edit | edit source]
R.I. Hutagalung
Department of Animal Sciences, University Pertanian Malaysia, Serdang, Selangor, Malaysia Introduction-2'
'Carbohydrate residues available'
'References
References[edit | edit source]
1. W.R. Stanton and A. Wallbridge, "Fermented Food Process," Process. Biochem., 4: 45-51 (1969).
2. B.H. Webb, R.l. Hutagalung, and S.T. Cheam, "New Developments in Palm Oil Waste Treatment," Proc, Agro-industrial Wastes Symposium, Kuala Lumpur, Malaysia, 19-20 Sept. 1975 (BRIM), pp.191-202.
3. B.H. Webb. R.l. Hutagalung, and S.T. Cheam, "Palm Oil Waste as Animal Feed: Processing and Utilization," in W. Earp and W. Newall, eds., International Developments in Palm Oil (Inc. Soc. Pltrs., Kuala Lumpur, Malaysia, 1976), pp.125-145.
4. R.l. Hutagalung, C.C. Chang, S. Jalaludin, and B.H. Webb, "Evaluation of Agricultural Products and BYproducts as Animal Feeds: IV. The Value of Processed Oil Palm Sludge for Chicks," Malay. Agric. Res., 4: 53-60 11975).
5. R.l. Hutagalung, B.H. Webb, and S.T. Cheam, "Potential of Palm Oil Mill Effluent as Animal Feed" (20th Annual General Meeting and Conference of the Malaysian Veterinary Association, Petaling Jaya, Malaysia, 25-27 Oct.1975),
6. R.l. Hutagalung and P.H. Tan, "Utilization of Nutritionally Improved Cassava by Nutrient Supplementation and Microbial Enrichment in Poultry and Pigs," in Proc. Fourth Symposium Internat. Soc. Trop. Root Crops (Cali. Colombia, 1 976), pp. 255-262.
7. R.l. Hutagalung, "Non-traditional Feedingstuffs for Livestock," in C. Devendra and R.l. Hutagalung, eds., Feedingstuffs for Livestock in South East Asia (MSAP, Kuala Lumpur, Malaysia, 1978), pp. 259-288.
8. S. Muttamara and N,C. Thanh, "Production of Feedingstuffs from Waste Waters," in C. Devendra and R.l. Hutagalung, eds., Feedingstuffs for Livestock in South East Asia (MSAP, Kuala Lumpur, Malaysia, 1978), pp. 245-258.
9. C.K. John, "A Medium for Isolation and Cultivation of Hevea Latex Bacteria," d. Rubber Res. Inst Malay., 20: 285-289 ( 1968).
10. C.K. John, "Studies on Some Gelling Properties of Hevea Latex Serum," J. Rubber Res. Inst. Malay., 23: 317-326 (1973).
11. P.R. Kulkarni, "Recovery of Algae Grown on Rubber Effluents," in W.R. Stanton, ea., Waste Recovery by Micro-organisms (Ministry of Education, Kuala Lumpur, Malaysia, 1972), pp. 118128.
12. R.N. Muthurajah, C.K. John, and H. Lee, "Development on the Treatment of Effluent from New Process SMR Factories," Proc. Rubber Res. Inst. Malay. Pltrs. Conference (Kuala Lumpur, Malaysia, 1973), pp. 402-411.
13. C.K. John, "Non-rubber Constituents of Hevea Latex and Their Possible Utilisation," in W.R. Stanton, ea., Waste Recovery by Micro-organisms (Ministry of Education, Kuala Lumpur, Malaysia, 1972), pp. 110117.
14. C.K. John, "Agro-industrial Wastes and Their Utilisation in Malaysia," in W.R. Stanton and E.J, DaSilva, eds., GIAM V Global Impacts of Applied Microbiology (UNEP/Unesco/lCRO Panel of Microbiology Secretariat, Kuala Lumpur, Malaysia, 1978), pp.148-155,
15. J.S. Lowe, "Bowl Sludge as a Fertilizer," Pltrs. Bull. Rubber Res Inst. Malay., 96: 91 -98 (1968).
16. E. Pusparajah, G. Haridas, N.K. Soong, and P. Zeid, "Utilisation of Effluent and Bowl Sludge from Natural Rubber Processing," in Proc. Agro-indust. Wastes Symposium I BRIM, Kuala Lumpur, Malaysia, 1975), pp.170-177.
17. P,H. Tan, K.R. Pillai, and D.J. Barry, "Possible Utilization of Rubber Factory Effluent on Cropland," in Proc. Internat. Rubber Conf. (BRIM, Kuala Lumpur, Malaysia, 1975).
18. N. Diokno-Palo, "Vinegar Production from Sugared Coconut Water," in W.R. Stanton, ea., Waste Recovery by Micro-organisms (Ministry of Education, Kuala Lumpur, Malaysia, 1972), pp.103-106.
19. P.M. Jayatissa, E.E. Jeya Raj, A.S. L. Tirimanna, and U.M. Senanayake, "Utilisation of Waste Coconut Water to Obtain a Potable Spirit," in W.R. Stanton, ea., Waste Recovery by Microorganisms (Ministry of Education, Kuala Lumpur, Malaysia, 1972), pp.107-109.
20. R. Satchuthananthavale and V. Satchuthananthavale, "Biological Coagulation of Latex," Ouart. J. Rubber Res. Inst. Ceylon, 48: 147-153 (1971).
21. R.l. Hutagalung, B.H. Webb, and S. Jalaludin, "Evaluation of Agricultural Products and Byproducts as Animal Feeds: I. The Nutritive Value of Pineapple Bran for Chicks," Malay. Agric. Res, 2:3947 (1973).
22. R.l. Hutagalung and T.W. Chey, "Evaluation of Agricultural Products and By-products as Animal Feeds," in Proc. Conf. Malay Food Self-sufficiency (UMAGA, Malaysia, 1976), pp. 261 -272.
23. F.S, Kwan, R.l. Hutagalung, and M.l. Djafar, "Evaluation of Chemically Treated Pineapple Silage for Goats," in C. Devendra and R.l. Hutagalung, eds., Feedingstuffs for Livestock in South East Asia (MSAP, Kuala Lumpur, Malaysia, 1978), pp. 86-88.
24, A. Kamari, A.Z. Idrus, and Z. Merican, "Waste Problems in the Pineapple Industry," in Proc. Agroindustrial Wastes Symposium (BRIM, Kuala Lumpur, Malaysia, 1975), pp. 203-212.
25. C.W. Hesseltine, "A Millennium of Fungi, Food and Fermentation," Mycologia, 57: 149-197 (1965).
26. C.K. John, "Biological Coagulation of Hevea Latex Using Waste Carbohydrate Substance," J. Rubber Res. Inst. Malay., 19: 286-290 (1966),
27. A.J. Nell and D.H.L. Rollinson, "The Requirement and Availability of Livestock Feed in Indonesia," in Supporting Livestock Planning (working paper of UNDP/FAO Project/lNS/ 72/009,1974),
28. H.K. Lim, "Fodders and Feedingstuffs in Malaysia," Malay. Agric. J., 46: 63-149 (1967).
29. V.F. Hew, "Problematic Aspects of Carbohydrate Source Used for Pigs in Malaysia," in C. Devendra and R.l. Hutagalung, eds., Feedingstuffs for Livestock in South East Asia (MSAP, Kuala Lumpur, 1978), pp.117-190.
30. B.L. Nestel, "World Animal Production and Feed Supplies," in Proc. Conference on Animal Feeds of Tropical and Subtropical Origin (Tropical Products Institute, London, 1975), pp. 15-21.
31. M. Grace, Processing of Cassava, Agricultural Services Bulletin No. 8 (FAO, Rome,1971).
32. W.D. Gray and M.O, Abou-el-seoud, "Fungal Protein for Food and Feeds: I I I. Manioc as a Potential Crude Raw Material for Tropical Areas," Econ. Bot., 20: 251-255 (1966).
33. K. F. Gregory, A.E. Reade, G. L. Khor, J.C. Alexander, J.H, Lumsden, and G. Losos, "Conversion of Carbohydrates to Protein by High Temperature Fungi," Food Technol., 30: 30-35 (1976).
34. R.l, Mateles and S.R. Tannenbaum, eds., Single-Cell Protein (MIT Press, Cambridge, Mass., USA, and London, 1968).
35. R. Kihlberg, "The Microbe as a Source of Food," Ann. Rev. Microbiol., 26: 427-466 (1972).
36. J.T. Worgan, "World Supplies of Proteins from Unconventional Sources," in J.W.G. Porter and B.A. Rolls, eds., Proteins in Human Nutrition (Academic Press, New York, 1973), pp. 47-74.
37. J.T. Worgan, "Protein Production by Micro-organisms from Carbohydrate Substrates," in J.G.W. Jones, ea., The Biological Efficiency of Protein Production (Cambridge University Press, Cambridge, UK, 1973), pp. 339-361.
38. J.T. Worgan, "The Efficiency of Technological Systems of Food Production," in A.N. Duckham and J.W.G. Jones, eds., Food Production and Consumption (North-Holland Publishing Co., Amsterdam, Netherlands, 1976), pp. 216-229.
39. F. Nartey, "Aflatoxins of Aspergillus flavus Grown on Cassava," Physiol. Plantarum, 19: 818-822 (1966).
40, E.J. Brook, W.R. Stanton, and A. Wallbridge, "Fermentation Methods for Protein Enrichment of Cassava, "Biotechnol. Bioeng., 11: 1271-1284 (1979),
41. J. Strasser, J.A. Abbott, and R.F. Battery, "Process Enriches Cassava with Protein," Food Eng., 42: 112-116 (1970).
42. A. Spicer, "Protein Production by Micro-fungi," Trop. Sci., 13: 239-250 11971).
43. A. Spicer, "Proteins from Carbohydrates," Chem. Brit., 9: 100-103 11973).
44. A.E. Reade, and K.F. Gregory, "High-Temperature Production of Protein-Enriched Feed from Cassava Fungi," Appl. Microbial., 30: 897-904 (1975)
45. G.L. Khor, J.C. Alexander, J. Santos-Nuñez, A.E. Reade, and K.F. Gregory, "Nutritive Value of Thermo-tolerant Fungi Grown on Cassava," J. Inst. Canad. Sci. Technol. Aliment., 9: 139-143 11976).
46. G.L. Khor, J.C. Alexander, J.H. Lumsden, and G.J. Losos, "Safety Evaluation of Aspergillus fumigatus Grown on Cassava for Use as an Animal Feed," Canad. J. Comp. Med., 41: 428-434 (1977),
47. H.Y. Yeang, "Protein Enrichment of Tapioca by Fermentation" (unpublished report, University of Malaya, Kuala Lumpur, Malaysia, 1973).
48. W.E. Trevelyan, "The Enrichment of Cassava with Protein by Moist-Solid Fermentation," Trop. Sci.. 16: 179-194 (1974).
49. C.C. Chah, C.W. Carlson, G. Semeniuk, US. Palmer, and C.W. Hesseltine, "Further Investigation and Identification of Growth Promoting Effects of Fungus-Fermented Soybeans for Broilers," Poultry Sci., 55: 975-981 11976).
50, P.H. Tan, Nutritional and Safety Evaluation of Fermented Cassava as Animal Feed (thesis, University of Malaya, Kuala Lumpur, Malaysia, 1977),
Production of microbial protein for feed from banana rejects[edit | edit source]
Mary Arlene P. Saquido, Verma A. Cayabyab, and Flordeliz R. Uyenco
Natural Science Research Center, University of the Philippines, Quezon City, Philippines Introduction'
'Review of the literature'
'Materials and methods'
'Direct enzymatic fermentation
Introduction[edit | edit source]
One of our major scientific and technological advances has been in the area of harnessing the activities of micro-organisms. For years it has been known that numerous kinds of yeasts, fungi, and bacteria have a direct relation, either favourable or unfavourable, to operations such as brewing, wine-making, and cheese-making. These have emerged from small-scale or family arts to the present industrial scale. Only in the past few decades, however, have the advantages of exploiting microbial activity been fully appreciated, owing to advances in biochemistry,
One of the most recent applications of micro-organisms is in the search for additional sources of protein. The increasing world population results in a rising demand for protein for both human and animal consumption. The demand for protein is certain to become serious with overexploitation of the sea and the use of most of the available arable land as the rapid growth in population continues. We are therefore faced with the problem of finding new sources of protein that will not require agricultural land or costly and tedious means of production. The escalating prices of traditional protein ingredients for animal feeds - animal and plant proteins such as fish, meat, and soybean meals - have intensified the problem.
Micro-organisms as a source of protein are one solution to this problem. The single cell of a microorganism is a perfect protein factory. Under controlled conditions in a fermentor, the culture of single cells can effect a highly efficient transformation of simple substances into protein. Land use is negligible and the gain in time is great, because of the fast rate of reproduction by micro-organisms, Emil Mrak of Davis, California, has pointed out that a 1,000 lb steer can yield 1 lb of new protein per day, and 1,000 lb of soybeans can yield 80 lb of new protein per day, but the corresponding figure for 1,000 lb of yeast is 50 tons per day (1).
TABLE 1. Macromolecular Composition and General Properties of Micro-organisms
|
Bacteria |
Yeast |
Fungi |
Algae | |
| Doubling time (hours) Crude protein | 1-3 | 2-6 | 5-12 | 6-24 |
| (% dry cell weight) | 40-80 | 40-60 | 30-45 | 40-50 |
| Nucleic acids (%) | 8-20 | 5-15 | 6-13 | 45-51 |
| Carbohydrates and fats (%) | 10-30 | 10-40 | 10-45 | 34.6-45 |
| Ash content (%) | 4-10 | 4-10 | 4-10 | 5-8 |
| Temperature range (°C) | 22-55 | 25-40 | 25-50 | 25-32 |
| pH range | 5-7 | 3-5 | 6-8 | 6.9-9.6 |
a. Percentage G.
Interest in microbial protein production is increased because micro-organisms can utilize waste materials that cause pollution problems and are sanitary hazards. Agricultural waste is a renewable resource of great variety and potential. In recent years the use of wastes like bagasse, rice straw, rice hulls, manure, and starchy residues as substrates for growing microbes has been studied. If the use of these materials is industrially developed, a vast bulk of them could be rendered economically useful, and this would help control pollution and eliminate some waste-disposal problems as well.
Protein of microbial origin, called single-cell protein (SCP), or microbial protein, can be derived from a variety of micro-organisms, both unicellular and multicellular - namely, bacteria, yeasts, fungi, or microscopic algae. The macromolecular composition and general properties of these organisms are shown in table 1. These potentially important food substances are not pure proteins but are, rather, dehydrated cells consisting of mixtures of proteins, lipids, carbohydrates, nucleic acids and a variety of other non-protein nitrogenous compounds, vitamins, and inorganic compounds. Microbial protein is a nontraditional protein, it is not a palatable, desirable food and must be incorporated directly or indirectly into other foods.
Review of the literature[edit | edit source]
The use of waste products, specifically agricultural wastes such as rice straw, manure, and bagasse, has been the object of current studies on microbial protein production. Dunlap (2) stated that an agricultural waste, to be a useful substrate for production of microbial protein, must meet the following criteria: it should be non-toxic, abundant, totally regenerable, non-exotic, and cheap, and able to support rapid growth and multiplication of the organisms resulting in a biomass of high quality.
Rejects of Cavendish bananas (Muse cavendishii Lamb.), also locally known as tumok in the Philippines, constitute one of the major agricultural wastes in areas where this variety of banana is farmed for export. The Philippines is one of the top ten banana producing countries in the world (3). In 1975 alone, a total of 406,927 kg of the Cavendish variety, valued at US$35.25 million, were exported (4). This variety is grown and intensively cultivated in southern Mindanao. It has been reported that the yearly banana export constitutes only about 80 to 90 per cent of the total produce from about 22,000 hectares of land in Davao planted with this variety intended for export (5). The remaining 10 to 20 per cent is rejected because the size does not meet export standards. Aside from their wide use as dessert fruit, the rejected bananas may be used in cakes, muffins, soup, fried chips, or flower bud vinegar and in many other ways. The rejects are also used as animal feed (3), but most of them are simply thrown away, posing a sanitary hazard. Therefore, studies on the use of banana rejects for production of microbial proteins for feeds were begun.
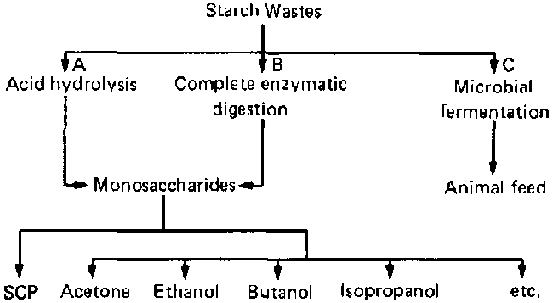
Different methods of converting starch wastes into useful products are shown in figure 1. There are two major processes: chemical conversion of the substrate by treatment with dilute acid in the presence of heat (A), and enzymatic hydrolysis, either through the direct action of selected groups of fungi and yeasts or through the use of highly active commercial enzyme preparations (B). In either case, the starch is hydrolysed into lower saccharides, predominantly glucose, which in turn are used either as raw material for chemical industries or as substrates for micro-organisms in the production of microbial proteins for human or animal consumption. Both of these processes are two-step reactions; i.e., the hydrolysis of the substrate is an entirely different step from the propagation of microbial cells or the chemical process. The present study is directed towards the improvement of the fermentation process involving the overall transformation of the starch to biomass through a one-step reaction (C) instead of the traditional two-step reaction.
FIG. 1. Utilization of Starch Wastes
Yeast is currently the most commonly used organism in the production of biomass, probably because it is already accepted both in the human food and animal feed industries (6). Yeast-based processes are the farthest advanced towards commercial production, followed by bacterial processes (7).
Yeasts have many convenient characteristics, such as the ability to use a wide variety of substrates such as hexoses, pentoses, and hydrocarbons (8; 9); susceptibility to induced and genetic variation (10), ability to flocculate (11); and high nutritional value (12; 13). However, attention has often been drawn to the fact that yeasts appear to be deficient in essential sulphur amino acids (14; 15). Nevertheless, this deficiency can be corrected by the addition of synthetic methionine, as shown in several studies done by Yañez et al. (12) and Harris et al. (16).
Until recently, the yeast most commonly employed has been Candida utilis grown on molasses (17) and sulphite liquor (18), but other species have been used successfully with various substrates such as methanol (19), whey (20), and hydrocarbons (21; 22). A study of Candida utilis grown on rye grass straw hydrolysate by continuous fermentation was reported by Han and Anderson in 1974 (23). In that study, the workers obtained cell densities of about 4 g/litre of medium. In North America some 25 per cent of sulphite liquor solids are made into yeast, representing a production of about 50,000 tons per year. The present use is mainly for animal feed supplements, the normal recommendation for mixed poultry feed being about 50 lb dried yeast per ton of feed. A factory in Taiwan produces food yeast from cane sugar molasses with a daily capacity of 40 tons of dried yeast (24).
A limited number of bacterial species have been grown specifically for food purposes, Recently, these organisms have been used extensively for SCP production on hydrocarbons such as petroleum, gas oils, and alkanes. Cellulomonas and Alcaligenes faecalis have been used in symbiotic fermentation studies using cellulose substrates (25-27). Although bacteria have a slight advantage over the other microorganisms as a food source because of their higher growth rates and relatively higher protein content and sulphur-containing amino acids (28), they have been objected to because of their size, which makes harvesting difficult without the use of flocculants or thickeners (29).
Algae processes are still short of full-scale development because they are limited by the requirements for light over a major portion of the year and for a continuous supply of CO2 or other carbon source 17). In Taiwan, however, plants for the production of Chlorella feeds using methane generated from manure are now in operation.
The production of fungal protein is far behind; it has not been scaled up to commercial level yet (6). Fungal proteins have not been considered for industrial SCP processes until recently, when studies on their potential as SCP proved that they exhibit growth rates comparable to those of yeasts and that crude protein contents in excess of 50 per cent can be achieved. Fungi have the ability to provide form and texture (30), and hence can be harvested with ease; also, the cost of production may be reduced. Like algae, fungi generally have low nucleic acid content, and accordingly, the dangers of kidney stones and gout are not great even without processing the biomass to lower nucleic acid content (7). Another advantage is that microfungi can prosper on a variety of carbohydrates, although growth rates vary considerably with different substrates (31).
Most species of fungi produce a range of carbohydrate-hydrolysing enzymes (31), but the amounts of the enzymes vary enormously between different organisms and more particularly between strains. Aspergillus niger reportedly produces large amounts of alpha-amylase and alpha-(1,6)-glucosidase, but less cellulase. Only a few fungal species exhibit problems of sucrose assimilation (or inversion}, and growth rates on these carbohydrates are usually similar to rates on glucose. Since carbohydrates are the main carbon sources of organisms, it would be reasonable to predict that fungal growth and yeast growth on bananas, which are largely composed of carbohydrates, would be substantial.
A comparison of the protein composition of micro-organisms and traditional sources of protein shows that microbial proteins are comparable to animal and plant proteins. Although it has been customary to regard animal proteins as nutritionally superior to vegetable proteins (including microbial protein), investigations have shown that many plant proteins in appropriate mixtures with one another, or with small quantities of animal protein, have a high biological value.
Many feeding trials have been carried out in animals with yeast and other microorganisms to assess their value as a source of protein and vitamins. The majority of animal feeds consist essentially of three components - in descending order of magnitude, cereals, high-protein ingredients, and mineral/vitamin/drug supplements (32). SCP is evaluated in terms of its ability to replace one or more of the high-protein sources in existing feeds (soybean, fish, meat, and blood meals and poultry offals) wholly or in part. Shacklady (32) reports that the normal protein requirement of laying and breeding hens is met when yeast at a dietary level of 12 to 14 per cent forms the sole high-protein source. Yeast also performed well in rations for turkeys at levels of up to 10 per cent of the total diet. Pigs were fed for five successive generations with yeast SCP replacing 10 per cent of the protein requirement with good results, and yeast SCP has been used successfully as the sole high-protein source in the rations of young beef animals.
Roberts (33) showed that Escherichia coli grown in aerated culture on a simple medium and heat dried was a very good protein supplement for rats and chicks. Micro" fungal proteins, when used at levels of 10.5, 21.0, and 42.0 per cent for feeding rats and chicks, showed results comparable to those obtained in animals fed casein (34).
The economic feasibility of any SCP process depends on its being able to produce a protein feed supplement of comparable quality and at a competitive price with alternative protein feed supplements such as soybean meal or fish meal. In the economic analysis of small-scale SCP processes from wastes, several factors have to be considered. Substrate costs can probably be minimal and in some cases may be considered negative, such as definite costs involved in the use of acid hydrolysis in the conversion of cellulose to glucose. Capital costs of the process can be reduced if low-technology procedures are used. In the Philippines, where extensive supplies of cellulosic and carbohydrate wastes are available, operating costs can also be reduced because of lower labour costs,
On the other hand, considerable costs may be involved in the collection of waste materials from food factories or agricultural feedlots located far from the SCP plant. Low-technology fermentation probably will produce SCP that is of a more variable composition than that produced by high-technology (controlled) fermentations using well-defined substrates, as practiced in developed countries. Extensive toxicological and acceptability tests will have to be performed before the product is approved for sale, and it is likely to command a lower price in the market.
A number of processing industries in South-East Asia release effluents that are rich in fermentable substrates, and this has raised interest in SCP production. Microbial upgrading of solid wastes is becoming increasingly attractive in view of stricter environmental regulations and the unacceptability of alternative treatment methods. Although it is still too early to come up with any detailed feasibility studies, it is evident that local markets for protein feed supplements do exist to replace the currently imported soybean and fish meals. The exploitation of our agricultural wastes for microbial protein production will greatly minimize, if not eliminate, the immense cost of waste pollution control.
Materials and methods[edit | edit source]
Substrate
Ripe and unripe Cavendish bananas in slurry form, used as substrate for fermentation, were prepared as follows: A known weight of bananas (whole fruit including peels) was chopped into small pieces and pureed with distilled water in a blender to make a slurry with a banana-to-water ratio of 1:3. The following supplements were added: (NH4)2SO4, 4 g/litre KH2PO4, 2 g/litre; MgSO4, 1 g/litre; and calcium pantothenate, 4.5 mg/litre. The pH of the slurry was between 4.5 and 4.9.
The Organisms
Four strains of Aspergillus niger (UPCC 3701, 3026, 3450, and 3809), two strains of Aspergillus foetidus (UPCC 3702 and 3448), and mixed cultures of Endomycopsis fibuligera (UPCC 2407) with Candida utilis (UPCC 2074) and of A. foetidus (UPCC 3448) with A. niger (UPCC 3809) were used as test organisms. Fungi were maintained on Ozapek Dox agar slants and yeasts on yeast malt agar slants at 29° to 30°C. These organisms were obtained from the culture collection of the University of the Philippines Natural Research Center.
Preparation of the Inoculum
Primary Inoculum
A.niger and A. foetidus were cultivated on Ozapek Dox agar slants, while E. fibuligera and C. utilis were cultivated on yeast malt agar slants. After three or four days of incubation at 29 to 30 C, spore suspensions of the organisms were transferred aseptically to bottles containing the supplemented substrate solidified with agar. This was done to acclimatize the organisms to the substrate. The inoculated bottles were incubated at 29° to 30°C for three or four days. The spore suspension prepared from these bottles was used as the primary inoculum.
Secondary Inoculum
The secondary inoculum was prepared by adding 5 ml of the primary inoculum to 10 ml of sterile banana slurry in a 500-ml Erlenmeyer flask, which was then shaken for 24 hours. After that, 60 ml of sterile medium was added, and shaking was continued for another 24 hours. Then 80 ml of fresh, sterile substrate was added, to make up a total volume of 150 ml, and the flask was again shaken for 24 hours The resulting culture was used as inoculum.
For succeeding fermentation runs, to minimize the time lag between runs, the inoculum was prepared by inoculating 150 ml of substrate with 10 to 15 ml of primary inoculum and shaking it for 24 hours. For fermentations on a larger scale, the inoculum was scaled up correspondingly.
Batch Culture Fermentation
Fermentation was carried out for 24 hours in a 5-litre reactor vessel (Marubishi Ltd., Japan) with a working volume of 2.5 litres. The pH was maintained at between 4.0 and 5.0 by automatic addition of NH4OH. Air flow rate was controlled from 0.5 to 5.0 litres/min, and agitation speed was regulated from 200 to 600 rpm to maintain the dissolved oxygen concentration above 1 ppm. Changes in pH, sugar concentration, and biomass concentration were noted at regular intervals (every four hours). Foam was controlled by the automatic addition of an antifoaming agent, while temperature was maintained at approximately 30°C.
Fermentation runs were carried out on a larger scale in a 14-litre Microferm fermentor (New Brunswick Sci. Co., USA) with a working volume of 7 litres. It has automatic pH, dissolved oxygen, temperature, and foam control. Maximum air flow rate values of 16 litres/min and agitation speeds up to 1,000 rpm can be attained. A 10 per cent v/v inoculum size was used.
Analytical Procedure
A 10 ml portion of every sample taken was centrifuged. The supernatant was collected and analysed for sugar content by means of the Somogyi-Nelson method of reducing. sugar determination (35; 36). The volume of residue was noted, and this was washed three times with 0.9 per cent saline solution and dried to constant weight at 60° to 80°C in a vacuum oven in aluminium foil boxes previously dried to constant weight. The dried samples were analysed for their crude protein content by the micro-Kjeldahl method (37).
Results and Discussion
The main pulp of the Cavendish banana contains a considerable amount of carbohydrates, mostly starch and reducing sugars, but is low in protein. The peels have a crude fibre content of 2.08 per cent (unripe) and 1.93 per cent (ripe). The extremely large reserves of polysaccharides make the banana rejects a potential source of SCP.
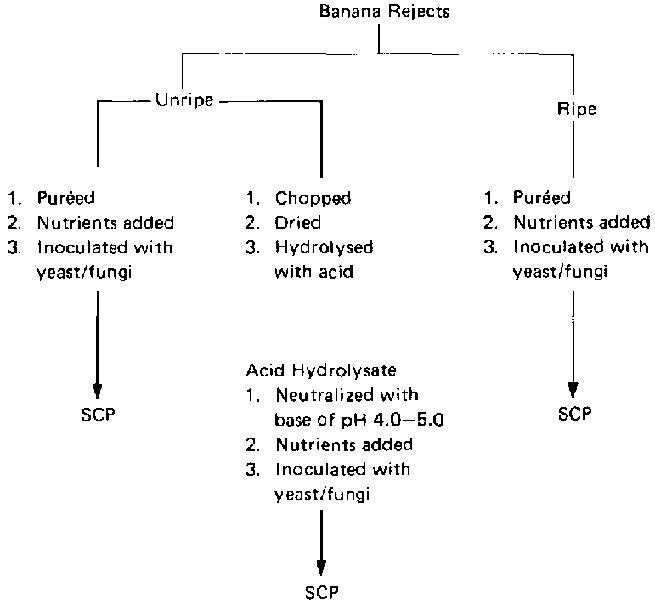
Two methods were proposed for using the banana rejects (fig. 2). The first involves treating the bananas with dilute acid in an autoclave, which results in the breakdown of starch and cellulosic materials into simple sugars. The hydrolysate obtained is used as substrate for SCP production, giving rise to a product consisting wholly of fungal mycelia or yeast cells. The second method is the direct enzymatic fermentation by selected fungi and yeasts of slurry prepared from the bananas. The product obtained in this method consists of yeast cells or fungal mycelia plus unhydrolysed banana residues. All of the investigations carried out made use of the whole banana fruit (pulp and peel), as it would not be economical if only the pulp or the peels were used.
Acid Hydrolysis
Studies on the acid hydrolysis of bananas were conducted. The effects of several factors, namely, acid concentration, banana-to-water ratio, and time (duration) of hydrolysis, on sugar yield were studied. Hydrolysis was carried out at 121°C because results of studies done in our laboratory on the acid hydrolysis of rice straw 138) as well as those reported in the literature (24) showed that the yield of sugar at this temperature was 30 per cent higher than the yield at 100°C at all the acid concentrations used
FIG. 2. Use of Banana Wastes for the Production of Single-Cell Protein (SCP) by Microbial Processes
Results show that maximum reducing-sugar yield (as glucose) from dried, unripe bananas was obtained when banana diluted with water to a 1:2 ratio was treated with 4 per cent H2SO4 for 30 minutes, or with 2 per cent H2SO4 for 1 hour at 121°C. Dried, ripe bananas had lower sugar yields when subjected to the same conditions of hydrolysis. This is because starch is converted to simpler sugars as the banana ripens, and acid hydrolysis of the ripe banana results in further degradation of these sugars to produce furfural and other degradation products that could be inhibitory to microbial growth. Hence, ripe bananas do not need to be hydrolysed in order to prevent the production of toxic by-products. Much of the reducing sugars present in ripe bananas can be directly used by micro-organisms for SCP production.
TABLE 2. Fermentation Data on Some Yeasts and Fungi Grown on Banana Slurry by the Batch Culture Method
|
Protein Content (N x 6.25) of Product (%) |
Crude Crude Protein Yield (g/ml) (%) |
Conversion of Sub strafe to Crude Protein | ||
| Aspergillus niger (UPCC 3450) | ripe | 19.25 | 0.0048 | 17.20 |
| unripe | 28.02 | 0.0065 | 15.82 | |
| A. niger (UPCC 3026) | ripe | 30.67 | 0.0076 | 32.90 |
| unripe | 19.88 | 0.0046 | 15.23 | |
| A. niger (UPCC 3701) | ripe | 27.51 | 0.0085 | 40.43 |
| unripe | 22.25 | 0.0054 | 21.01 | |
| A. niger (UPCC 3809) | ripe | 25.31 | 0.0065 | 26.29 |
| unripe | 23.85 | 0.0066 | 19.41 | |
| A. foetidus (UPCC 3448) | ripe | 27.83 | 0.0083 | 45.11 |
| unripe | 20.88 | 0.0055 | 14.82 | |
| A. foetidus (UPCC 3702) | ripe | 26.58 | 0.0063 | 31.50 |
| unripe | 21.75 | 0.0050 | 13.89 | |
| Endomycopsis fibuligera | ||||
| (UPCC 2407) and Candida | ripe | 30.08 | 0.0070 | 24.05 |
| utilis (UPCC 2074) | unripe | 30.26 | 0.0076 | 16.02 |
| A. foetidus (UPCC 3448) | ripe | 41.30 | 0.0082 | 57.85 |
| and A. niger (UPCC 3809) | unripe | 32.09 | 0. 0080 | 31.36 |
a. Average values of several fermentation runs.
Studies on acid hydrolysis were later abandoned because this process does not appear to be economically feasible. Considering the amount of acid, heat, water, and time required for the process, this method is an expensive step in the production of SCP. Thus, the emphasis in the present research project is confined to direct enzymatic fermentation by micro-organisms.
Direct enzymatic fermentation[edit | edit source]
One phase of this research that is being investigated is concurrent enzymatic starch hydrolysis and yeast cell multiplication through the use of a mixed culture. This process would be cheaper than starch hydrolysis by acid followed by yeast propagation and is accomplished by inoculating the banana slurry with equal volumes of Endomycopsis fibuligera, a mycelial yeast that produces an amylase capable of breaking down starch into glucose, and Candida utilis, a known food yeast that cannot use starch directly but can use glucose.
Growth of these organisms on unripe banana substrate increased the crude protein content of the final product (yeast cells and unhydrolysed banana residues) to an average value of 30.26 per cent. On ripe bananas, average final crude protein content was 30.08 per cent. The fermentation data are shown in table 2. The relevant equations are:
protein yield =[% crude protein]/100 x product yield (final dry weight)
conversion efficiency = [protein yield] / initial dry weight x 100
During fermentation, considerable weight loss is observed with unripe banana substrate, while there is a slight decrease in dry weight when ripe bananas are used.
Dry weight measurements, however, cannot be used to follow the growth of the organisms since the samples are a mixture of microbial cells and unhydrolysed banana residues that cannot be separated. The behaviour of dry weight measurements can be interpreted in terms of a balance between microbial growth, which tends to increase dry weight, and hydrolysis of the banana slurry, which tends to decrease dry weight of the product, The final product is lower in dry weight because of incomplete conversion to microbial cells of the hydrolysed banana substrate. Part of it is metabolized to other products such as CO2 and residual sugars or is converted to energy (as ATP), which is required for hydrolysis.
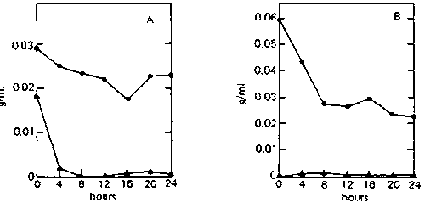
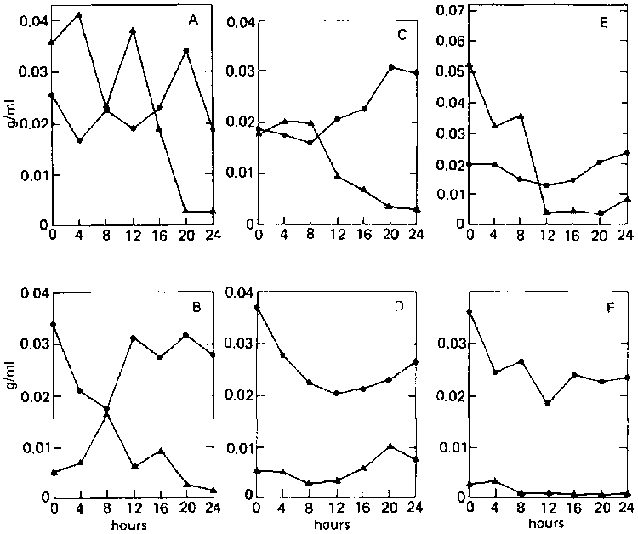
On both ripe and unripe bananas, reducing-sugar concentration in the supernatant remains very low (less than 0.003 g/ml) throughout the fermentation period (fig. 3). This is because C. utilis immediately utilizes the sugars released by E. fibuligera from the banana substrate.
Fig, 3. Fermentation Patterns of a Mixed Culture of Endomycopsis fibuligera (UPCC 2047) and Candida utilis (UPCC 2074) on Ripe and Unripe Banana Slurry. A: On ripe bananas. B: On unripe bananas.
^ = reducing-sugar concentration (as glucose, g/ml).
· = dry weight.
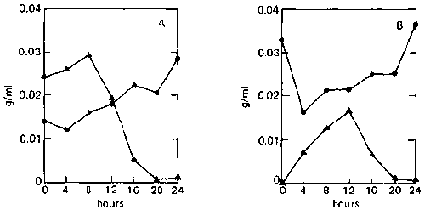
Two other organisms used mixed culture are A. niger 3809 and A. foetidus 3448. A. niger is known to produce large amounts of amylases and also cellulases; A. foetidus produces only small amounts of amylases; and both can use glucose equally well. In contrast to the mixed culture of two yeasts, reducing sugar is found to accumulate in the medium. When unripe bananas are used, rapid enzymatic hydrolysis occurs during the initial growth phase (fig. 4B). Sugar concentration increases rapidly during the first 12 hours (0.0002 to 0.0167 g/ml, or an 80-fold increase), which is accompanied by a sharp decline in dry weight. Dry weight then increases with time with a corresponding decrease in sugar concentration.
In the case of ripe bananas, despite the high concentration of sugar initially present (0.0242 9/ml), slight hydrolysis still occurs during the first eight hours, causing the sugar concentration to increase to 0.0292 g/ml with a corresponding decrease in dry weight (fig. 4A). This may mean that the enzymes are not inhibited at this sugar level. Rapid utilization of the sugar follows, accompanied by an increase in dry weight. Accumulation of sugar in the medium is not surprising, as both organisms are amylase-producing.
FIG. 4. Fermentation Patterns of a Mixed Culture of Aspergillus niger (UPCC 3809) and A. foetidus (UPCC 3448) on Ripe and Unripe Banana Slurry. A: On ripe bananas.
B: On unripe bananas.
^ = reducing-sugar concentration (as glucose, g/ml).
· = dry weight.
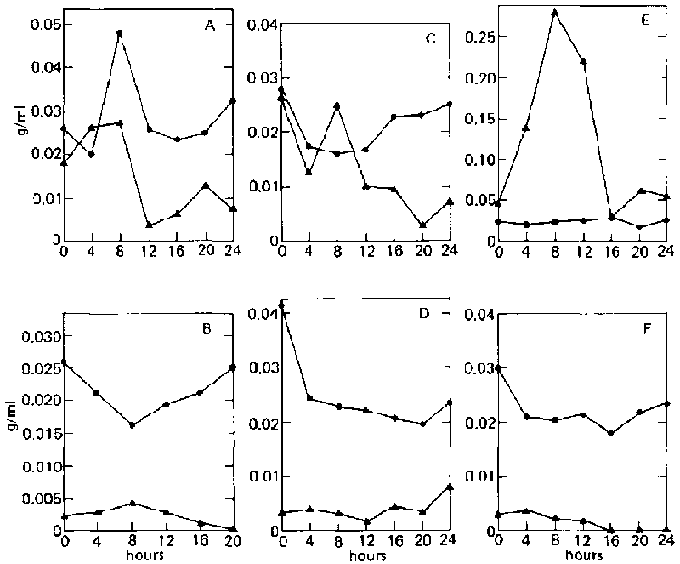
The second phase under investigation is the direct fermentation of bananas into microbial protein by one organism alone. Different isolates of A. niger and A. foetidus were used in this study. Results are shown in table 2.
When A. niger 3809 is used alone, final dry weight is lower for both ripe and unripe bananas, although the same changes in dry weight during fermentation are observed as in the mixed culture of the two fungi (fig. 5A and B). The same general behaviour of reducing sugar in the medium is also observed, With ripe bananas, two peaks are observed, one at 4 and the other at 12 hours. However, the increase in sugar concentration is small (0.0355 to 0.0412 and 0.0380 g/ml). In the case of unripe bananas, the peak is reached 8 hours after inoculation (0.0052 to 0.0165 g/ml, representing a threefold increase in sugar concentration), with a smaller peak at 16 hours (0.0093 g/ml).
When A. foetidus 3448 is used alone on ripe bananas, a very slight peak in sugar concentration appears after 4 hours (0.0178 to 0.0201 g/ml) accompanied by a slight decrease in dry weight (fig. 5C and D). In the case of unripe bananas, final dry weight is lower, with the dry weight changing gradually during the fermentation, as shown by a smooth, rounded curve, and the peak in sugar concentration occurring only after 20 hours (0.0053 to 0.0101 g/mg, or a twofold increase), which probably indicates slow hydrolysis of the banana. This confirms an earlier statement that the amylase of A. foetidus 3448 is weaker than that of A. niger 3809.
FIG. 5. Fermentation Patterns of Single-Organism Cultures of Aspergillus niger (UPCC 3809) and A. foetidus (UPCC 3448 and 3702) on Ripe and Unripe
Banana Slurry.
A: A. niger 3809; ripe.
B: A. niger 3809; unripe.
C: A. foetidus 3448; ripe.
D: A. foetidus 3448; unripe.
E: A. foetidus 3702; ripe
F: A. foetidus 3702; unripe
^ = reducing-sugar concentration (as glucose, g/ml).
· = dry weight.
FIG. 6. Fermentation Patterns of Single-Organism Cultures of Aspergillus niger (UPCC 3701, 3450, 3026) on Ripe and Unripe Banana Slurry.
A: A. niger 3701; ripe.
B: A. niger 3701; unripe.
C: A. niger 3450; ripe.
D: A. niger 3450; unripe.
E: A. niger 3026; ripe.
F: A. niger 3026; unripe.
^ = reducing-sugar concentration (as glucose, g/ml).
· = dry weight.
We can conclude that the use of A. foetidus 3448 and A. niger 3809 in combination is better than using either one alone, because the crude protein content of the final product and the efficiency of conversion of substrate to protein is higher when the mixed culture is used, whether with ripe or unripe bananas.
A. foetidus 3702 behaves in a similar manner to A. foetidus 3448 on both ripe and unripe bananas (fig. 5E and F). There is very slight hydrolysis of the substrate, indicated by the absence of sharp peaks in reducing-sugar concentration in the medium. A. foetidus 3448 may even be slightly better than A. foetidus 3702 because more sugar accumulates in the medium when the former is used. It has also been observed that the crude protein content of the products and conversion efficiency of the two organisms are similar,
When the three remaining strains of A. niger (3701, 3450, and 3026) are compared, final dry weight is higher than the initial weight on ripe bananas for A. niger 3701, lower for A. niger 3450, and approximately the same for A. niger 3026 (fig. 6). A. niger 3026 produces the highest peak in reducingsugar concentration after 8 hours (0.0465 to 0.2789 g/ml, for a sixfold increase), while the dry weight hardly changes. For A. niger 3701, a small peak occurs at 8 hours, with the dry weight surprisingly reaching a peak at this point also. This may be attributed to sampling error With A. niger 3450, a rapid decline in sugar concentration prior to the occurrence of a peak that is lower than the initial value is accompanied by a decrease in dry weight.
Two peaks in sugar concentration are always observed, a large peak during the initial growth phase and a smaller peak after most of the sugar has been used up. This may be attributed to increased activity of the starch-hydrolysing enzymes when the sugar concentration drops to a low level or to decreased growth rate of an organism that has probably reached the stationary phase while enzyme activity remains the same, leading to accumulation of the released sugar in the medium. This is not observed with A. niger 3809.
On unripe bananas, the final dry weight is lower for all three organisms. No appreciable peaks in sugar concentration are observed, indicating that the hydrolysis taking place is just enough to support microbial growth. A. niger 3809 may have stronger amylase activity on this substrate, since more sugar is released Into the medium (0.0052 to 0.165 g/ml).
When the crude protein content of the final product and conversion efficiency are compared, growth on ripe bananas generally produces higher values than on unripe bananas. A. niger 3701 has the highest average crude protein content on ripe bananas (27.51 per cent). The value for A. niger 3026 was taken from one fermentation run only and so cannot be interpreted as the highest. A. niger 3701 also has the highest conversion efficiency. On unripe bananas, A. niger 3450 has the highest protein content, but A. niger 3701 has the highest conversion efficiency.
For all the fermentation runs performed, the final product is almost always lower in dry weight than the starting material when unripe banana slurry is used as substrate. In some cases, reduction in weight of more than 50 per cent is observed. In the case of ripe bananas, final dry weight may be higher or lower. In contrast to the mixed culture of yeasts, accumulation of reducing sugar in the supernatant is always observed sometime during the fermentation when fungi are used. This drops to low levels as the fermentation continues.
A mixed culture of A. foetidus 3448 with A. niger 3809 appears to be the best on ripe and unripe bananas, followed by a single culture of A. niger 3701 and a mixed culture of E. fibuligera 2047 with C. utilis 2074. However, definite conclusions cannot be drawn until a more direct measurement of starch utilization has been carried out. A method of starch analysis is being studied, and we hope analyses can be completed in the near future. An indirect method of following microbial growth - analysis of the protein content during fermentation - is also being studied. Relative nutritive values of the different types of microbial protein produced cannot be compared at present because analyses of the amino acid contents and toxicological tests have yet to be carried out.
Results of this research so far have demonstrated the possibility of growing fungi and yeasts on banana waste with relatively high crude protein content. However, it is not comparable to that of current protein sources such as plant proteins and commercial animal feeds (e.g., soy meal, 45 to 50 per cent; fish meal 60 to 65 per cent). It has also been shown that no preliminary chemical treatment is necessary as long as the appropriate organisms are used. Further studies will be conducted in an attempt to increase the protein content and conversion efficiency.
References
1. R. Acker, "Global Impacts of Applied Microbiology," ASM News, 44: 102-104 11978).
2. C.E. Dunlap, "Production of Single-Cell Protein from Insoluble Agricultural Wastes by Mesophiles," in S.R. Tannenbaum and D.l.C. Wang, eds,, Single-Cell Protein ll (MIT Press, Cambridge, Mass,, USA, and London, 1975), pp. 244-262.
3. J.C. Trinidad, "The Philippines Is Still Asia's Banana King," The Republic, 1 (23): 5 (1977).
4. J.L. Castro, "Banana, Country's Sixth Biggest Export Product," The Times Journal, 4: 78 11976).
5. R.L. Locsin, "Cavendish Bananas Are Suitable for Ketchup," Business Day, 8: 54 11974).
6. F.K, Imrie and A.J. Vlitos, "Production of Fungal Protein from Carob (Ceratonia siliqua L.)," in S.R. Tannenbaum and D.l.C. Wang, eds., Single-Cell Protein II (MIT Press, Cambridge, Mass., USA, and London, 1975), pp. 223-243.
7. E.S. Lipinsky and J.H. Litchfieid, "Single-Cell Protein in Perspective," Food Technol., 2815): 16 (1974).
8. H. Suamalainen and E. Cura, in A.H. Rose and J.S. Harrison, The Yeasts [Academic Press, London and New York, 1971), pp. 3-60.
9. M. Kanazawa, "The Production of Yeast from n Paraffins," in S.R. Tannenbaum and D.l.C. Wang, eds., Single-Cell Protein II (M IT Press, Cambridge, USA, and London 1975), pp. 438453.
10. H. Heslot, "Some Genetic Aspects of Petroleum Yeasts" [Paper presented at Unesco/UNEP/ ICRO Regional Microbiology Training Course, Bangkok, Thailand, 26 March-18 April, 19761.
11.T.P. Labuza, "Cell Collection: Recovery and Drying for SCP Manufacture," in S.R. Tannenbaum and D.l.C. Wang, eds., Single-Cell Protein ll (MIT Press, Cambridge, Mass., USA, and London, 1975), pp. 69104.
12. E. Yañez, D. Ballester, N. Fernandez, V. Gattos, and F. Mönckeberg, "Chemical Composition of C utilis and the Biological Quality of the Yeast Protein," J. Sci. Food Agric., 23: 581-586 (1972).
13. R. Bressani, "The Use of Yeast in Human Foods," in R.l. Mateles and S.R. Tannenbaum, eds., SingleCell Protein (MIT Press, Cambridge, Mass., USA, and London, 1968), pp. 90-121.
14. J.T. Worgan, in J.G.W. Jones, ea., The Biological Efficiency of Protein Production (Cambridge University Press, Cambridge, UK, 1973), pp. 339-361.
15. G.E.N. Nelson, R.F. Anderson, R.A. Rhode, M.C. Shekleton, and H.H. Hall, "Lysine, Methionine, and Tryptophan Content of Microorganisms: II. Yeasts," Appl. Microbiol., 8: 179-182 (1960).
16. E.E. Harris, M.A. Hannan, and R.R. Marquardt, "Production of Food Yeast from Wood Hydrolysates: Nutrient Requirements," Ind. Eng. Chem., 40 (11): 2068-2072 (1948).
17. E.R. Dawson, "The Cultivation and Propagation of Bakers Yeast," Chem. Ind., 793-797 1952).
18. G.C. Innskeep, A.J. Wiley, J,M. Holdenberg, and L.P. Hughes, "Food Yeast from Sulfite Liquor," Ind, Eng. Chem., 43: 1702 (1951).
19. T. Oki, K. Kuono, A. Kitai, and A. Ozaki, "New Yeasts Capable of Assimilating Methanol," J. Gen. Appl. Microbiol., 18: 295-305 (1972).
20. V.E. Graham, D.C. Gibson, H.W. Dlemmer, and J.M. Naylon, "Increasing the Food Value of Whey by Yeast Fermentation," Canad. J. Tech., 31: 85-109 (1953).
21. K. Yamada, J. Takahashi, Y. Kawabata, T. Okada, and T. Onihara, "SCP from Yeast and Bacteria Grown on Hydrocarbons," in R.l. Mateles and S.R. Tannenbaum, eds., Single-Cell Protein (MIT Press, Cambridge, Mass., USA, and London, 1968), pp. 193-207.
22. A. Champagnat, C. Vernet, B. Laine, and J. Filosa, "Biosynthesis of Protein-Vitamin Concentrates from Petroleum," Nature, 197: 13 (1963).
23. Y.W. Han and A.W. Anderson, "The Problem of Rice Straw Waste: A Possible Feed Through Fermentation," Econ. Bot., 28 (3): 338-344 (1974).
24. C. Rainbow and A.H. Rose, eds., Biochemistry of Industrial Microorganisms (Academic Press, London and New York, 1963).
25. V.R. Srinivasan and M.B. Fleenor, "Fermentative and Enzymatic Aspects of Cellulose Degradation," in Developments of Industrial Microbiology (American Institute of Biological Sciences, Washington, D.C., USA, 1972), pp. 47-53.
26. Y.W. Han and V.R. Srinivasan, "Isolation and Characterization of a Cellulose Utilizing Bacterium, " Appl. Microbiol., 14: 1140 (1968) .
27. Y.W. Han and V.R. Srinivasan, "Purification and Characterization of Beta-Glucosidase of A. faecales," J. Bacteriol., 100: 1355 (1969).
28. H.J. Bunker, "Sources of Single-Cell Protein: Perspective and Prospect," in R.l. Mateles and S.R. Tannenbaum, eds., Single-Cell Protein (M IT Press, Cambridge, Mass., USA, and London, 1 968), pp. 6778.
29. R. Dabbah, "Protein from Microorganisms," Food Technol., 24 (6): 35 (1970).
30. A.E. Humphrey, "Product Outlook and Technical Feasibility of SCP," in S.R. Tannenbaum and D.l.C. Wang, eds., Single-Cell Protein ll (MIT Press, Cambridge, Mass. USA, and London, 1 975), pp. 1-23.
31. C. Anderson, J. Longton, C. Maddix, G.W. Scammell, and G.L. Solomons, "The Growth of Microfungi on Carbohydrates," in S.R. Tannenbaum and D.l.C. Wang, eds., Single-Cell Protein II /MIT Press, Cambridge, Mass., USA, and London, 1975), pp. 314-329.
32. C.A. Shacklady, "Value of SCP for Animals," in S.R. Tannenbaum and D.l.C. Wang, eds,, Single-Cell Protein ll (MIT Press, Cambridge, Mass., USA, and London, 1975), pp. 489-504.
33. L.P. Roberts,Naturo (London) 165:494 (1950).
34. I.F. Duthie, "Animal Feeding Trials with a Microfungal Protein," in S.R. Tannenbaum and D.l.C. Wang, eds. Single-Cell Protein 11 (MIT Press, Cambridge, Mass., USA, and London, 1975), pp. 505544.
35. J. Somogyi, "Notes on Sugar Determination," J. Biol. Chem., 195: 19-32 (1952).
36. N. Nelson, "A Photometric Adaptation of the Somogyi Method for the Determination of Glucose," J. Biol. Chem., 153: 375-380 (1944).
37. C.H. Perrin, "Rapid Modified Procedure for Determination of Kjeldahl Nitrogen," Analyt Chem., 25 (6): 968-971 (1953).
38. V.A. Cayabyab, "Growth Characteristics of Candida utilis var. thermophila and Candida tropicalis on Rice Straw Acid Hydrolysis in Batch Cultures" (M.S. thesis, Department of Botany, University of the Philippines, Quezon City, Philippines, 1976).
Potential alternative energy sources in the South Pacific[edit | edit source]
References
A model of bioconversion of aquacultural residues for aquaculture
Introduction'
'Materials and methods'
'Results and discussion '
'Conclusions'
'References-1
M.K. Jogia
University of the South Pacific, Suva, Fiji
Like most developing countries throughout the world, Fiji is feeling the pinch of the energy crisis with increasing shortages and rising prices of one of the modern sources of energy, fossil fuels. Alternative sources of energy in developing countries in the South Pacific need to be investigated. What is needed is a source that would be economically viable, easily accessible, and inexhaustible for a reasonable period of time. For countries such as Fiji, where there is considerable emphasis on farming in the rural areas, utilization of natural products (e.g., sugar cane, cassava, wood) for energy resources should be encouraged if the factors involved in processing them are favourable.
In 1977, total fuel consumption in Fiji was about 760,000 tons, or 1,280 kg per person, 50 per cent of which was imported commercial fuel and the remainder indigenous, non-commercial (1). Industry (mostly sugar processing) accounted for about half of all energy use, with one quarter used for transportation and the other quarter for business and domestic purposes. Commercial energy consumption was 30 per cent electric and 70 per cent non-electric. Nearly all of the transport energy and 90 per cent of the electricity generated were obtained from petroleum fuels. Table 1 illustrates the sources of energy, and table 2 the use of these sources by the different sectors of the community (1).
The Government of Fiji, itself concerned about the consequences of further increases in the price of oil, let alone the possibility of not being able to purchase it at any price, has initiated a team of consultants to conduct a feasibility study on the production of ethanol from cassava. Incidently, the governments of Papua New Guinea and the Solomon Islands have also carried out a similar study. The terms of reference for the Fiji study include consideration of small-scale production: "to investigate as a special case the feasibility of cassava-based ethanol production on a small scale (5 ha crop or less) on remote islands for use in electricity generation and outboard motors .. ." (2).
TABLE 1. Estimated Gross Energy Consumption, Fiji, 1977
|
Quantity |
Energ |
Total |
% | |
|
(million kg) |
(1,000 |
(1010 Btu) | ||
| Petroleum fuels | 218 | 44.3 | 966 | 46.5 |
| Liquid petroleum gas | 2 | 48.0 | 10 | 0.5 |
| Coal | 23 | 25.8 | 59 | 2.8 |
| Bagasse (dry) | 350 | 18.7 | 655 | 31.5 |
| Wood (oven dry) | 208 | 18.6 | 387 | 18.7 |
| Total | 2,077 | 100.0 |
a. Calculated on the basis of an average consumption of 350 kg/person (urban, 80 kg/person x 37.3 per cent, and rural, 510 kg/person x 62,7 per cent), and a mid-1977 population of 595,000.
b. About 760,000 tons coal equivalent.
TABLE 2. Percentages of Gross Energy Consumption by Sector and Fuel Type, 1977
|
Imported Fuel |
Local Fuel |
Total | |||
|
Petroleum |
Coal |
Bagasse |
Wood | ||
| Industrial | 10.3 | 2.8 | 31.5 | - | 44.6 |
| Transport | 24.7 | - | - | - | 24.7 |
| Household, including subsistence level | 6.2 | - | - | 18.6 | 24.8 |
| Commercial,government, and miscellaneous | 5.8 | - | - | 0.1 | 5.9 |
| Total | 47.0 | 2.8 | 31.5 | 18.7 | 100.0 |
Considering that there is a significant amount of fertile virgin land available not only in Fiji but in neighbouring countries as well (e.g., the Solomon Islands, the New Hebrides) that could be cultivated, it would be beneficial not only to the members of the rural community, but to the country as a whole, if it were practical to use cassava as an alternative source of energy. Cassava is relatively easy to grow, the villagers know how to plant it, and it does not take years for a crop to be ready for harvest.
It has been reported that the content of starch in cassava varies over the year (3). Perhaps local crops could be studied to determine the amounts of starch in cassava over a period of time.
The Fiji Sugar Corporation is also investigating the extraction of ethanol from sugar cane juice, although the possibility of using molasses had been considered earlier (4).
In determining the development of a plant to produce ethanol from either sugar cane or cassava, or both, the Government of Fiji would also have to consider that there is undeveloped land available in most of the outer islands of Fiji, and this may tend to indicate that a cassava-processsing plant would be in Fiji's best interests, although initially it may not be as economical as a sugar cane factory. It should also be noted that conditions with regard to availability of land, terrain, etc., differ throughout the 11 countries represented by the University of the South Pacific; thus, while it might be favourable to establish a cassava plant in Fiji, it would not be so in Tuvalu, for example.
The source of energy that has been used extensively over the years by the rural communities in the regional countries has been wood (5). The net fuel wood resource in Fiji in 1980 was conservatively estimated as 463 tons of coal equivalent, which is triple previous use and 17 per cent above commercial energy consumption (6). As an alternative source of energy, a wood-based system to produce a combustible gas (producer gas, wood gas) for stationary engines could be developed to complement liquid fuel from alcohol.
Current research on energy in the Department of Chemistry at the University of the South Pacific has been concentrated on the formation of biogas. The anaerobic digestion of a mixture of field grasses (mainly Axonopus compressus, Eleusine indica, and Digitaria longiflora), water hyacinths (Eichhornia crassipes), and seaweeds (Gracaleria, Verrucosa, and Sargassum) has been investigated as a potential input supplement for biogas digesters currently in operation in Fiji and elsewhere in the South Pacific (7).
In conclusion, it is noted that alternative sources of energy in the South Pacific can be obtained from sugar cane, cassava, and wood. However, taking into account the socio-economic factors involved, it may not be possible to single out any one source as the only choice.
References[edit | edit source]
1. P. Johnston, at the Seminar on Woods as an Alternative Energy Resource, University of the South Pacific, Suva, Fiji, 3-4 July 1978.
2. Terms of Reference for Ethanol Study, as approved by Cabinet Sub-committee, Fiji Government.
3. V. Yang, W.N. Milfont, Jr., A. Scigliano, C.O. Massa, S. Sresnewsky, and S.C. Trinidade, "Casava Fuel Alcohol in Brazil," in Proceedings of the 12th Intersociety Energy Engineering Conference (19771,1 : 4453.
4. South Pacific Island Business News, October 1979, p. 7,
5. S. Siwatibau, A Survey of Domestic Rural Energy, Energy Use and Potential in Fiji, report to the Fiji Government and the international Development Research Centre (IDRC), Ottawa, Canada (University of the South Pacific, Suva, Fiji, 1978).
6. P. Johnston, A Preliminary Study of Fuel Wood for Rural Electrification in Fiji (Commonwealth Regional Consultive Group on Energy, New Delhi, India, 1978).
7. R.K. Solly, The Production of Biogas from Water Hyacinth (Commonwealth Science Council/ United Nations Environment Programme, New Delhi, India, 1978).
A model of bioconversion of aquacultural residues for aquaculture[edit | edit source]
H. Hirata and S. Yamasaki
Kagoshima University, Kagoshima, Japan
Introduction[edit | edit source]
This paper will describe an attempt to maintain a steady-state zooplankton community in a feedback culture system. A transparent, round, 550-litre tank connected to a 150-litre zigzag stream unit was used for multi-species culture of Brachionus plicatilis and Tigriopus japonicus. The water in the system was recirculated about 20 times per day by air-lift pumps. The animals were fed frozen baker's yeast daily, and faeces were removed from the bottom of the stream and transferred into a sludge activator. Marine chlorella cultured in the sludge were then fed back to the zooplankton. Approximately 10 to 20 per cent of the animals were harvested each day of the 480-day experiment. The relative proportion of B. plicatilis and T. japonicus in the community was maintained at a steady-state ratio of 82:18 by body volume. The proportion of nauplii, copepodites, and adults of T. japonicus was also constant during the culture period at a ratio of 28:47:25. The food conversion efficiency was calculated to be 26.7 per cent.
Recently, because of rapid development of mass production of zooplankton as food for cultured fish and prawn larvae, two serious ecological problems have emerged: energy loss in feeding and water pollution from excretion 11-3). For example, about 10 kcal of yeast must be fed to produce 1 kcal of rotifers by culturing (4); thus, about 90 per cent of the energy source is lost. Also, rotifers produce large amounts of faeces, causing gradual pollution of the culture medium. This type of water pollution is becoming common. Self-purification is carried out smoothly in natural sea water but is almost impossible in an accumulation microcosm because of over-biodeposition by the cultured animals. Therefore, supplements must be added to promote the energy flow (5).
Our experiment was conducted to determine how to maintain a steady-state zooplankton community in an accumulation microcosm by a feedback culture system. Steady-state zooplankton communities have also been studied by some other microcosm researchers (6-11). The idea of the feedback system was initiated in about 1930 and has been developed in the field of electronic circuits (12). Since the term "feedback" is convenient, it has been used in several scientific fields, e.g., biochemistry, nerve physiology, and ecosystem research (13). The feedback system discussed here refers to feeding and excretion, then excretion to feeding again to regulate the energy flow in the system.
Materials and methods[edit | edit source]
Figure 1 is a flow chart of the feedback culture system. Baker's yeast is fed to zooplankton; organic matter from the faeces and the uneaten food is mineralized by bacteria into inorganic nutrients for algae; then the algae are fed to the zooplankton. Marine zooplankton organisms, Brachionus plicatilis and Tigriopus japonicus, were cultured together as the consumers. Microalgae, Chlorella sp. (probably Chlorella saccharophila var. saccharophila, as reported by Tsukada and co-workers [14] ) and Nitzschia spp., and macro-algae, Enteromorpha intestinalis, were cultured as the producers at the beginning of the experiment. Ten to 15 species of marine bacteria that grew naturally in the tanks acted as decomposers.
FIG 1. Feedback Culture Systems: (a) Mono-feedback System, and (b) Multifeedback System. The monotype system is used for the culture of rotifers, which are herbivorous. The more complicated multi-type system is suitable for carnivorous animals. A-1, herbivorous animals; A-2, carnivorous animals; B-1 and B2, decomposers; C-1 and C-2, algae reproduced by excess nutrients in the culture system.
FIG. 2. Process of the Rotifer Culture in the Feedback System. Biodeposits and water are reused for chlorella culture as by-product nutrients.
Two round polycarbonate tanks were used for the zooplankton culture: tank A for the feedback experiment and tank B for batch culture. Tank A was connected to a 150-litre zigzag stream unit, but tank B was not (fig 2). The water in tank A was recirculated to the stream unit by an air-lift pump at a rate of about 20 times per day, resulting in a water current in the stream of approximately 1 m/mint The feedback producer, marine chlorella, was cultured in two 60-litre transparent tanks used alternately at two-day intervals. Every three or four days the faeces and uneaten food were siphoned from the bottom of the stream unit and transferred to the decomposer tank (modified from Fujiwara et al. [15] ).
Water removed from tank A after harvesting of the zooplankton and from the decomposer tank was transferred to the chlorella culture tanks. Macro-algae (E. intestinalis) were grown, together with the zooplankton, in the zigzag stream unit.
The experiments were conducted for 480 days, from 12 March 1976 to 4 July 1977, under laboratory conditions. Water temperatures ranged from 16.4° to 30.5°C and were maintained within tolerable limits by electric heaters that came on when the temperature fell below 17°C. In addition to natural illumination, white-beam fluorescent lamps (eight 40-watt and sixteen 20-watt lamps) were used to maintain a 15-hour light and 9-hour dark photo-period (16).
During the first 30 days, 552 g of activated sludge composed of soycake particles and dried yeast (17), 608 g of heads and bony parts of fish, 60 g of marine chlorella, 18 g of diatoms, and 308 g of baker's yeast were supplied to tank A and to the stream unit. After the fifty-fourth day of culture, 30 g of wet weight marine chlorella reproduced in the chlorella culture tanks was also supplied to the zooplankton daily as feedback food. The zooplankton population density, pH, and phosphate content were measured each morning before feeding. Special caution was taken to control the zooplankton density within a range of 50 to 100 individuals per millilitre by harvesting.
Results and discussion[edit | edit source]
Population Density of B. plicatilis in the Feedback Culture
Seasonal variations of the zooplankton population density are shown in figure 3; figure 4 shows the algal feedback rate; and water temperature, pH and PO4-P content are given in figure 5. Figure 6 shows algal productivity. The feedback rates were calculated as amount of chlorella fed times 100 divided by total food input ( kcal ).
FIG. 3. Population Densities of Zooplankton throughout the Culture Experiment in the Feedback Culture System
FIG. 4. Chlorella Feedback Rate - Calculated as the Amount of Chlorella Fed Multiplied by 100 and Divided by the Total Food Supplied (kcal)
FIG. 5. The Culture Medium in the Feedback Culture System.
A: pH.
B: water temperature.
C: Phosphate content.
The initial B. plicatilis population density was only 3.7 individuals per millilitre, but it increased about tenfold during the first month of culture. Population density was maintained at about 45/ml from day 30 to day 70 by daily harvesting. Beginning at day 70, when chlorella was fed back to the animals and E. intestinalis started to grow in the stream unit (fig. 4), the B. plicatilis population density increased to 60 to 65/ml. The density was then maintained at 57 to 65/ml until day 185. Thereafter the density fluctuated from about 50 to 75/ml through the winter season (days 195 to 365) because of the lower temperature (16° to 24° C).
After 365 days of culture, the B. plicatilis population density was increased by more feeding and less harvesting to about 100 individuals per millilitre as a trial. The density decreased gradually, however, to 40 to 50/ml during the last four months when the growth rate of algae decreased from about 700 to 200 kcal/day and PO4-P contents in the water increased from 2 to 5 mg/litre at the same time (see fig.6). On the basis of these results, we estimated that the optimum population density of B. plicatilis for maintaining a steady state in this system is 59.8 ± 5.4 individuals per millilitre.
FIG. 6. Total Amount of Algae Harvested (kcal) and Dissolved Phosphate Content during the Last Half of the Experiment
Population Density of T. japonicus in the Feedback Culture
The population density of T. japonicus was about 25 per cent that of B. plicatilis. The initial density of T. japonicus was only 1.1 individuals per millilitre; this was the lowest level in the experiment. The greatest density was 21.2/ml, observed on day 335. The density ranged from 6 to 17, averaging about 11/ml throughout the culture period. Fluctuations in T. japonicus population density followed a pattern similar to that of B. plicatilis, with the peak density occurring approximately 10 to 20 days later. The population density of B. plicatilis increased rapidly at approximately day 75, whereas T. japonicus density peaked near day 95 (fig. 3).
Population densities of T. japonicus nauplii were closely related to the amount of chlorella fed back, but were not related to the growth of E. intestinalis. The reverse was true for the copepodites of T. japonicus; i.e., they were affected by the growth of E. intestinalis but not by the amount of chlorella fed back. The adult T. japonicus population density was not affected by either of these factors, but was affected by the reproduction rate of B. plicatilis. This may be a result of the different feeding habits of T. japonicus in each developing stage (18).
Food Conversion Efficiencies
Efficiencies of food conversion in the feedback system are shown in figure 7. Two methods were used to calculate the rates - Z conversion:
[ zooplankton harvest x 100] / total food supplied
and Z+E conversion:
[ total zooplankton and E. intestinalis harvested x 100 ] / total food supplied
FIG. 7. Food Conversion Efficiencies during the Experiment, Calculated by Different Methods (see text)
All the data presented here come from measurement of caloric contents of materials by bomb calorimetry.
The Z conversion efficiency was only 5.2 per cent between days 11 and 20 of the experiment. It increased to 23.2 per cent between days 141 and 150 (fig. 7), and thereafter varied between 23.0 and 27.2 per cent. An interesting result was also observed for the Z+E conversion efficiency. Harvesting of E. intestinalis began on day 80, and the Z+E conversion efficiency increased gradually from 16.4 per cent at the first harvesting to 60.1 per cent between days 400 and 410. Thus, the total Z+E food conversion efficiency was 57.7 per cent.
Composition of the Zooplankton Community in Feedback and Batch Culture Tanks
The B. plicatilis and T. japonicus proportions of the zooplankton community, by body volume, are given in figure 8. Variations in the density proportions of each develop mental stage - nauplius, copepodite, and adult - of T. japonicus, in numbers of individuals per millilitre, are shown in figure 9. The balance between the two zooplankton species was maintained in a steady state throughout the 480-day culture experiment in the feedback system; i.e., the dominant species was always B. plicatilis (82.2 per cent), and T. japonicus made up only 16.8 per cent of the community. The highest ratio of B. plicatilis to T. japonicus was 92:8 on days 121 to 130, and the lowest was 68:32 on days 391 to 400.
The average proportions of T. japonicus developmental stages (nauplii:copepodites: adults) in the feedback culture system were constant at 28:47:25 throughout most of the 480-day experiment (fig. 9). During the last five months of the culture period, however, the copepodites gradually became dominant, and the proportion during the final ten days (days 471 to 480) was 18:66:16 (nauplius:copepodite:adult).
The succession of the zooplankton community in the feedback and batch culture tanks differs greatly (fig. 8). At the beginning of the experiment, the composition of the zooplankton population was approximately 50 per cent B. plicatilis and 50 per cent T. japonicus in the feedback system. The respective percentages were 98 and 2 in the batch culture tank because of a difference in the density during initial inoculation. Approximately 20 days after inoculation, the proportions were the same in both systems (82 per cent B. plicatilis and 18 per cent T. japonicus). This density was maintained until the end of the experiment in the feedback culture system (see fig. 8). The proportion of B. plicatilis in the batch culture tank decreased, however, after day 90, and all the zooplankton in the control tank disappeared after day 120.
The PO4-P contents of the culture water at day 120 were 2.4 mg/litre in the feedback and 5.1 mg/litre in the batch culture tank. The excess nutrients in the feedback system were used by the algae, marine chlorella, and E. intestinalis.
FIG. 8. Composition of the Zooplankton Community (Percentages of Tigriopus japonicus and Brachionus plicatilis). A: In the batch culture system. B: In the feedback culture system.
FIG. 9. Relative Proportions of the Different Developmental Stages - Nauplius, Copepodite, and Adult - in the Composition of the Tigriopus japonicus Population
Conclusions[edit | edit source]
Comparison of the results from the feedback and batch (the traditional method) culture systems of zooplankton shows the very significant role of producers (Chlorella and E. intestinalis) and decomposers in maintaining a steady state in an ecosystem. Throughout 480 days of culture, with almost no change of culture medium, the population of both B. plicatilis and T. japonicus was maintained in the feedback culture system. It is significant that the zooplankton in the batch culture tank totally disappeared after only 120 days of culture. A stable zooplankton community was maintained in the feedback system for the duration of the culture period, whereas the batch culture had deteriorated by day 90 (fig. 8).
At day 120 the inorganic phosphate content was only 2.4 mg/litre in the feedback culture system, whereas that of the batch culture was 5.1 mg/litre (figure 5). This indicates that successful mineralization of organic wastes and their subsequent utilization by algae were responsible for maintaining a favourable water quality in the feedback system.
The food conversion efficiency in the feedback system was 25.6 per cent for the duration of culture; this is much higher than that reported by other investigators (4, 19). The high conversion efficiency was a result of the feedback culture, since about 20 per cent of the total food supplied to the zooplankton was chlorella. This means that the recycling of energy in the feedback culture system gives a food saving of 20 per cent. It might be suggested, therefore, that the feedback culture system acted to purify water and to conserve energy.
The harvesting effect is also important in maintaining a steady state in the feedback culture. The results of our experiment suggest that it is possible to maintain a steady-state zooplankton population for long periods of time by harvesting in a feedback system. We hope to develop a simpler and more efficient feedback system for conservation in accumulation microcosms.
References[edit | edit source]
1. H. Hirata, "Zooplankton Cultivation and Prawn Seed-Production in an Artificial Ecosystem," Helgol. Wiss Meeresunters, 30: 230 (1977).
2. H. Hirata, "Principle of Feedback Culture System and Its Developmental Experiment," Yoshoku, 15 (1): 34 (1978).
3. H. Hirata, "Seed-Production of Prawn, Penaeus japonicus, with Reference to Application of Active Sludge Method," Food Ind. (Tokyo), 21 110): 29 (1978).
4. K. Fukusho, 0. Hare, and J. Yoshio, "Mass Production of Rotifer Fed Chlorella and Yeast in Large Tanks," Aquaculture, 24: 96 (1976).
5. O. Kinne, ea., Cultivation of Marine Organisms: Water Quality Management and Technology in Marine Ecology (John Wiley & Sons, New York, 1976), 3, part 1: 19-300.
6. R.J. Conover, "Cultivation of Plankton Populations," Helgol. Wiss. Meeresunters, 21: 401 ( 1970).
7. R.J. Conover and E.D.S. Corner, "Respiration and Nitrogen Excretion by Some Marine Zooplankton in Relation to Their Life Cycles," J. Mar, Biol. Assoc., (UK), 48: 49 (1 968).
8. Y. Kurihara, "Studies of 'Succession' in a Microcosm," Sci. Rep. Tohoku Univ., fourth series, 37:161 (1978).
9. Y. Kurihara, "Studies of the Interaction in a Microcosm," Sci. Rep. Tohoku Univ., fourth series, 37: 178 (1978).
10. J. Ringelberg, "Properties of an Aquatic Micro-ecosystem: 11. Steady-State Phenomena in the Autotrophic Subsystem," Helgol. Wiss. Meeresunters, 30:134 (1977).
11. J. Ringelberg and K, Kesting, "Properties of an Aquatic Micro-ecosystem: I. General Introduction to the Prototypes," Arch. Hydrobiol, 83: 1 (1978).
12. S. Inoue, M. Kotani, F. Tamamushi, and K, Toyama, Dictionary for Physics and Chemistry, 6th ed. (Iwanami Shoten, Tokyo, 1958), p.1132.
13. T. Yamada et al., Biological Dictionary, 2nd ed. (Iwanami Shoten, Tokyo, 1977).
14. O. Tsukada, T. Kawahara, and H. Takeda, "Good Growth of Chlorella saccharophilia on the Basis of Dry Weight Under NaCI Hypertonic Condition," Bull. Japan Soc. Sci. Fish., 40: 1007 (1974).
15. M. Fujiwara, Y. Itokawa, N. Sakuyama, T. Nakata, M. Kimura, and Y. Nishino, "A Method of Biological Purification of Waste Materials from Hospitals," in K. Arima, ea., Report on Purification of Environment by Microorganisms (Faculty of Agriculture, Tokyo University, 1975), pp. 178-179.
16. H. Hirata, "Preliminary Report on the Photoperiodic Acclimation for Growth of Chlorella Cells in Synchronized Culture," Mem. Fac. Fish. Kagoshima University (Kagoshima, Japan) 24: 1 (1975).
17. H. Hirata, A. Kanazawa, T. Yamamidori, and K. Yasuda, "Preliminary Studies on Sludgezation of SoyCake Particles and Yeasts," Mem. Fac. Fish., Kagoshima University (Kagoshima, Japan). 22:107 (1973).
18. H. Hanaoka, "Cultivation of Three Species of Pelagic Micro-crustacean Plankton," Nihon Purankuton Gakkaiho, 20: 19 (1973).
19. Y. Tanaka, "Tracing of Bio-deposits by Fish Culture," in Seawater Fish Culture and Self-Pollution, ed. Japanese Society of Scientific Fisheries (Koseisya Koseikaku Co., Tokyo, 1977), 21: 42-50.