Chapter 5 - Oilseed products and further processing[edit | edit source]
Oil for human consumption[edit | edit source]
Oils and fats constitute an essential part of the human diet. Together with proteins, carbohydrates, vitamins and minerals they are some of the main nutrients required by the human body. They are rich sources of energy, containing two and a half times the calories of carbohydrates (per unit weight). In addition to being a source of vitamins A, D, E and K, fats also contain essential fatty acids. Essential fatty acids cannot be manufactured by the body and must be obtained from the diet. Examples are linoleic and linolenic acids.
Refined vegetable oils, and foods rich in fats, are readily available in developed countries; they can be found in great variety on any supermarket shelf. As foods containing carbohydrate and protein are also readily available, the importance of fat as an energy source in developed countries is probably less significant than its role in supplying vitamins and essential fatty acids.
Human food products prepared from oilseeds in Africa[edit | edit source]
Culi-culi is a snack food made in Ghana from groundnuts, and is prepared from the de-oiled paste left over from the traditional village method for extracting oil from groundnuts. In this method, the groundnut kernels are reduced in size using a village hammer-mill or a pestle and mortar. The groundnut paste is put into a bowl and water is kneaded into it by hand. After the water has been added, the mixture is kneaded until the oil flows freely from the paste. The oil is decanted and used for cooking. The de-oiled paste is formed into rings and fried in oil to make culi-culi.
Peanut butter This method for preparing peanut butter was used in a domestic science class at ATC, Farafenni, Gambia.
- Examine the decorticated kernels carefully and remove stones and other trash by hand. Sieve the kernels to remove any small stones which may still be present. Make sure that the groundnuts have no trace of mould growth.
- Remove any fine dust from the surface of the kernels by wiping them with a cloth.
- Prepare a fire and roast the kernels in sand. This is done by using a shallow bowl, about 300 mm in diameter, with a layer of clean, washed sand about 10 mm deep at the bottom. The layer of sand helps to transfer the heat evenly to the kernels without burning them. The kernels must be continually stirred with the sand while they are being heated. The roasting takes about 10 min.
- After roasting, remove the sand from the kernels by shaking them in a finely meshed sieve. After the loose sand has been removed, wipe the kernels with a cloth to remove any sand clinging to their surfaces.
- Remove the skins from the kernels by rubbing the kernels together. Shake the kernels and skins together in a bowl so that the skins collect at the bottom. Carefully sort out the kernels and remove them from the bowl.
- Add any salt desired to the roasted kernels and pound them into coarse fragments in a pestle and mortar. Finally, use a rolling pin to crush the fragments to make peanut butter.
- Hand-operated disc mills or meat-mincing machines can also be used to grind the roasted peanut kernels into peanut butter.
Use of oil-cake in animal feeds[edit | edit source]
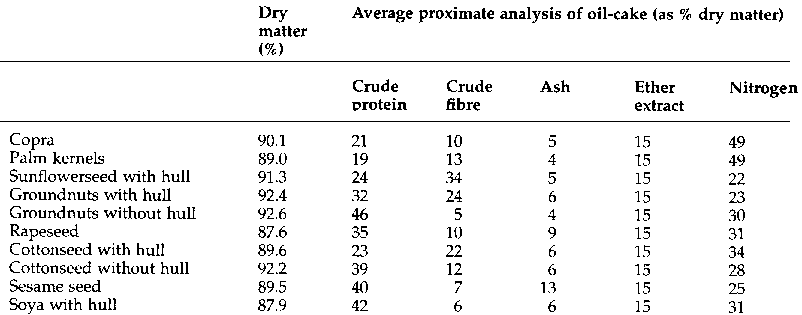
Oil-cake is the residue obtained after the greater part of the oil has been extracted from an oilseed. Oil-cakes are rich in protein and most are valuable food for farm animals. The typical composition of selected oil-cakes is given in Table 4. Oil-cake from large-scale oil mills is often an ingredient of compounded animal feeds. Some seeds, such as castor beans, yield oil-cakes unsuitable for direct incorporation into animal feed as they contain toxic substances.
It is only possible here to give a general account of the role of oil-cake in animal nutrition since blending optimum diets for different types of animals involves a number of considerations which are outside the scope of this book. Readers interested in obtaining further details on this subject are recommended to obtain NRI Bulletin 9 The small-scale manufacture of compound animal feed.
Acceptable feeds can be produced by blending about 30% of oil-cake from small expellers with other local ingredients such as cereals and bran.
Table 4 Typical composition of selected oil-cakes
Animal feeds consist of carbohydrates, fats and oils, and proteins, together with smaller amounts of minerals and vitamins which are essential for the proper functioning of body metabolic processes.
Most farm animals will develop with feeding systems consisting of a small range of components, or even a single component, but production levels may be low and if the nutrient levels in the feed are not balanced, those present in excess will be wasted. Optimum yields of meat, milk or eggs are obtained by feeding farm animals appropriate quantities of various raw material components.
The most important nutritional features of feed raw material are the amounts of usable energy and protein which they provide to various classes of livestock. In general, fats and carbohydrates are the main sources of energy, while proteins and minerals are necessary largely for the building and replenishment of body tissue. An adequate supply of protein is of particular importance for growing animals, which require a higher proportion of protein in their rations than mature animals.
Most farm animals have either ruminant or non-ruminant digestive systems. Ruminant animals such as sheep, cattle and goats have compound stomachs; they have some capacity to digest dietary fibre and to convert non-essential amino-acids in their diet into essential amino-acids through rumen microbial fermentation. The non-ruminant animals are monogastric (with single stomachs), such as pigs and poultry. Non-ruminants have a very restricted capacity to digest fibre. Essential amino-acids, in the correct proportions must be provided by their diet as they cannot be formed in the body from other compounds.
Roughage, concentrates and supplements are the main types of materials used for animal feeds. Roughage is made up of bulky fibrous plant materials, fresh, dried or ensiled, which are mainly suitable for feeding ruminant animals. Concentrates are non-fibrous, starchy or proteinaceous materials suitable for feeding to all kinds of animals. The main types of concentrates are cereals and cereal by-products, legumes, oil-cakes, dried starchy roots and tubers such as cassava and potatoes, animal and fish by-products such as meat, blood and bone meal, fish meal, and dried milk. Supplements are minerals, vitamins and medicaments which maintain animal health and ensure satisfactory feed conversion. Miscellaneous materials, such as grass and lucerne meals, dried sugar-beet pulp, molasses and carobs, are also used.
The quality of the protein in oil-cake is considerably higher than that of cereals but it tends to be of poorer quality than animal protein and, in particular, meatmeal or fishmeal protein. Proteins consist of chains of sub stances called alpha amino-acids linked to one another. Some 20 amino-acids are normally found in proteins but it is now known that only some of these are 'essential', in that sufficient of them must be present in the ration to meet the minimum nutritional requirements of the animal. The quality of proteins for nutritional purposes is determined by the proportions of the essential amino-acids present.
Oilseed proteins are normally deficient in one or more essential amino-acids. As a result they cannot provide adequate supplementation of the cereal proteins with which they are commonly used, and should be fed in conjunction with an animal protein, e.g. meatmeal or fishmeal, when given to monogastric animals.
When oilseed residues are used to raise the protein level of animal diets, they may be complemented by legume seeds, animal by-products and synthetic amino-acids. As well as cost, the major decisive factor for using a particular oilseed residue depends on the amino-acid composition of the cereal and cereal by-products incorporated in the diet. If, however, most of the energy is derived from root crops, then the oilseed residues will be required in greater quantities to contribute a higher proportion of protein.
Oilseed cake may make a significant contribution to the energy content of the diet, particularly when the oil content is high. Digestive disturbances, however, may result from the uncontrolled use of cakes rich in oil, or if the oil is unsaturated. Body fat may become soft and carcass quality may be lowered. Oilseed-cakes usually have a high phosphorus content but a low calcium content. They contain only negligible amounts of vitamin A-active materials, vitamin D and vitamin B12. The content of other group B vitamins is rather higher than that of pulses.
The fat in oil-cakes is also usually a good source of linoleic acid, which is essential for animal metabolic processes.
For oilseeds with fibrous husks, such as groundnuts and sunflower seed, the protein content of the oil-cake is higher if the husks are removed before the oil is extracted. Oil-cakes from oilseeds extracted with the husks on have a much higher crude fibre content than those from oilseeds from which the husks have been removed. Oil-cakes of high crude fibre content cannot be included in high proportions in feeds for non-ruminant animals.
Oilseeds may contain a number of toxic or undesirable substances, which may pass unaltered into the oil-cake. These substances may be natural constituents of the seeds, such as gossypol and cylopropenoid fatty acids in cottonseed, cyanogenetic glycoside in linseed, ricin in castor beans, sinigrin or sinalbin in mustard seed, saponin in shea nuts, the trypsin inhibitor in soyabeans, or toxic mould metabolites, such as aflatoxin, which may form if the seeds are allowed to spoil by moulds. Rubber seed, in addition to containing cyanogenetic glucosides and a chemical factor with gossypol characteristics, has a shell which causes irritation in the animal's gastrointestinal tract. The effect of some of these undesirable factors can be reduced by restricting the amount of the particular oil-cake present in the animal's diet, by heating or steaming the oil-cake, by extracting it with water, or by using oil-cake from decorticated seed. The processing, particularly if heat is involved, may result in a lowering of the digestibility and in denaturation of the protein, with a consequent lowering of its nutritive value.
If livestock are scarce in the locality where small-scale processing is being carried out, the local market for oil-cake my not be promising. The quantity of oil-cake produced using small-scale manual methods of extraction will usually be limited. Larger quantities will be produced from small oilseed expellers and it is possible for a market to be found for this material. Depending on the seed type, it may be possible to feed the residue directly to animals without further treatment. In the Cook Islands, copra cake from a small expeller has been sold to farmers as a pig feed supplement. In the Gambia, groundnut cake produced from a small expeller has replaced cake from a large-scale commercial mill in poultry rations used on a farm in a rural location. If a local market for the oil-cake cannot be found, it may still be worthwhile transporting it to a region where livestock ownership is more widespread. This will depend on sufficient quantities of the cake being produced.
Soap manufacture[edit | edit source]
Soap can be in short supply in rural areas of developing countries, but it may be made easily by mixing oils and fats with a solution of caustic soda in water. The process is simple but it needs to be done carefully. The oil, caustic soda and water used to make the soap have to be mixed together in the correct proportions. This is important because soap-making involves a chemical reaction between the oil and the caustic soda in the water. The oil and the caustic soda combine to make one product, the soap. In this reaction, a definite weight of oil reacts with a definite weight of sodium hydroxide If more caustic soda is used than is needed it will remain in the soap and produce a burning action on the skin.
Making the caustic soda solution has to be carried out carefully since caustic soda is very corrosive. All contact with the skin should be avoided. On accidental contact, the skin must be washed immediately with water. It is advisable to wear safety goggles, or face shields, and rubber gloves when making up the solution. Caustic soda solution reacts with zinc, tin, aluminium and brass very readily, so only utensils and containers made from iron, steel, wood or plastic should be used. When the caustic soda is dissolved in water, a considerable amount of heat is generated. It is very important that when mixing the caustic soda and water, the caustic soda must always be added to the water. Never attempt to add the water to the caustic soda. This will cause an explosive evolution of heat. It is also important to add the caustic soda to the water in small amounts, at a slow rate, so that the solution does not heat up much above 60°C. If the caustic soda is added too quickly, the solution can boil and produce irritating caustic steam and spray.
A number of things affect the soap-making process and the quality of the soap produced. The characteristics of the soap depend on the type and quality of the oil, and the amounts of caustic soda and water used to make it. The speed of the reaction between the oil and the caustic soda is influenced by the free fatty acid content of the oil, the temperature of the components before mixing, and how vigorously the mixing is done. High free fatty acid contents, vigorous mixing, and heat, tend to speed up the soap-making process.
Two groups of fats are used for soap-making. The first group, the lauric oils are obtained from the kernels of different types of palm. The most common oils in this category are coconut oil and palm kernel oil. They are known as lauric oils because they contain lauric acid as the major fatty acid. These fats make a hard soap which produces a fast-forming lather with a strong detergent action. The soap tends to have a harsh effect on the skin. Soaps made from lauric oils are used as salt water soaps.
Non-lauric oils are the other group of fats. These contain virtually no lauric acid and include a large selection of liquid oils such as olive oil, corn oil sunflower seed oil, groundnut oil, soyabean oil and cottonseed oil, as well as semi-solid fats such as palm oil and tallow. Soap made from the oils in this group produces lather more slowly than lauric oil soap, but the foam is longer-lasting, it has a milder detergent action and it has a gentler effect on the skin. Palm oil and tallow have a similar foaming action but the soaps produced are harder. Castor oil is also included in this category. It produces a hard but very soluble soap with poor foaming characteristics.
By making blends of these different types of oils, soaps with a range of characteristics may be produced.
The division of fats into lauric and non-lauric types is useful because it provides a simple way of calculating how much caustic soda should be added to the oil to make the soap.
The lauric oils need to be mixed with caustic soda in the ratio, by weight, of one part of caustic soda to six parts of oil, i.e. 1 kg of caustic soda needs to be added to every 6 kg of lauric oil.
The non-lauric oils require less caustic soda to make soap. They require the ratio by weight of one part of caustic soda to eight parts of oil, i.e. 1 kg of caustic soda needs to be added to every 8 kg of non-lauric oil.
These ratios use slightly less caustic soda than is theoretically needed to make soap from the oil which has been measured out. This it to make sure that no excess caustic is left in the finished soap.
When a mixture of oil types is used to make soap, the amount of caustic soda is calculated according to the amount of lauric and non-lauric oils present in the mixture. For example, a mixture of 2 kg of lauric oil and 8 kg of non-lauric oil would require 0.33 kg of caustic soda to react with the lauric fat, and 1 kg of caustic soda to react with the non-lauric fat. This adds up to a total of 1.33 kg of caustic soda for the 10 kg total weight of the fat mixture.
The caustic soda required to react with the fats is dissolved in water in the ratio of one part of caustic soda to two parts of water by weight. For example, 1 kg of caustic soda should be dissolved in 2 kg of water. The amount of water used is important as it affects the way in which the caustic soda reacts with the oil; also, excess water will evaporate from the finished soap when it is stored and cause it to distort and shrink.
The equipment needed for soap-making includes a weighing machine, volume measure, vessels to mix the caustic soda solution and make the soap in, stirring rods, soap moulds, and wire to cut the finished soap into tablets.
If they are available, kitchen or bathroom scales should be used to weigh out the materials. Volume measures such as those used in kitchens, or containers or bottles of known capacity, such as 1 litre, can also be used. A plastic bucket of about 8-10 l capacity is a useful vessel for mixing the soap ingredients in. Stirring rods can be made simply from suitable lengths of wood.
Soap moulds can be made from wood, but cardboard boxes are also suitable. A table can be adapted to make a soap mould by attaching removable lengths of wood, about 2.5 cm high, around the edge so that the block of soap can be moved forward for cutting after it has set. Larger moulds can also be made from boxes about 0.5 x 0.25 m in size which can be taken apart to release the soap after it has set. Lining the mould with thick plastic sheeting helps to stop the soap sticking in the mould and makes it easier to remove. Spreading a thin layer of petroleum jelly over the plastic sheet also helps to prevent the soap from sticking to the mould.
A length of cheese-wire or wire tuna-fishing line, attached at each end to wooden handles, can be used to cut up the soap blocks. For making soap tablets, a cutting board equipped with a guide-rail to align the soap bar at right angles to the cutting wire can easily be constructed from wood.
It is best to measure out the ingredients for soap making using a weighing machine such as bathroom or kitchen scales. However, if the caustic soda is sold in small packs of known weight, such as 1 kg packs, it will be possible to make soap without needing a weighing machine provided that volume measures, such as 1 l bottles, are available. The 1 kg of caustic soda from the pack is used as the basis for calculating the proportions of oil and water needed to make the soap. When measuring out the oil and water using a volume measure, it must be remembered that 1 l of water weighs 1 kg and that 1 l of oil weighs about 0.92 kg. Half litres can be measured using 1 l bottles of the same type; one of the bottles is filled with oil which is then poured into an empty bottle until the same level of oil is reached in each. Each bottle then contains 0.5 l. This process can be continued to measure 0.25 l by pouring the 0.5 l of oil into another empty bottle until the same level is reached in each. By making marks on the side of the bottles corresponding to the 0.5 l and 0.25 l levels obtained in this way, simple volume measures can be made.
To make soap from coconut oil using a 1 kg pack of caustic soda, first dissolve the 1 kg of caustic soda in 2 l of water (2 l of water weighs 2 kg) in a suitable plastic container.
Measure out 6.5 l of coconut oil (6.5 l of coconut oil weighs 6 kg) into a plastic bucket. The oil needs to be between 30 and 40°C; normal tropical day-time temperatures are usually satisfactory.
Allow the caustic soda solution to cool to about body temperature, 35-40°C, before adding it to the coconut oil. When the caustic soda solution has cooled, pour it slowly into the coconut oil, stirring the oil all the time so that the caustic soda solution is absorbed into the oil and does not separate from it. If the oil is slow in absorbing the caustic soda solution, add it in small amounts at a time and stir it well into the oil so that it is absorbed before more caustic soda solution is added. When all the caustic soda solution has been added keep stirring the mixture until it begins to thicken and patterns can be drawn on the surface with the tip of the stirring rod. The speed at which this thickening occurs is greatly influenced by the free fatty acid content of the oil used. The higher the free fatty acid content, the quicker the oil thickens. At this stage, before it thickens any further, the soap must be poured into moulds and allowed to harden for about 24 h. During this hardening time, the soap-making reaction continues and the soap will feel warm to the touch.
After the soap has hardened it should be removed from the mould and cut up into tablets using cutting wire. The soap tablets need to be stored for about two weeks before being used. This is to allow time for all the caustic soda to react with the oil. About 9 kg of soap will be obtained, equivalent to 90 soap bars of 100 g each.
In this soap-making method, the 1 kg of caustic soda was dissolved in 2 l of water. This makes a strong solution of caustic and helps to reduce the time that the soap takes to thicken when it is being mixed. However, if the oil has a high free fatty acid content, the soap may thicken too quickly and make it difficult to add all the caustic soda solution. If this happens, dissolve the 1 kg of caustic soda in 2.5 l of water for making the next batch of soap from the same oil. This makes a weaker solution and increases the time taken for the soap to thicken, but still makes a good quality soap with the advantage that 500 g of extra soap are made.
When oils with a very low free fatty acid content are used, the soap may take a long time to thicken. If the soap takes much longer than 45 min. to thicken, the caustic may not be absorbed very well. If this happens, it is best to heat the oil until it is just bearable to touch, about 60°C, before mixing in the caustic soda solution. This will decrease the time taken for the soap to thicken.
To make soap from a non-lauric oil using a 1 kg pack of caustic soda, use 8.75 l of the oil (about 8 kg) and follow the same method used to make soap from coconut oil.
Fragrances and colouring may be added just as the soap mixture starts to thicken, stirring them in well just before pouring it into the moulds. These fragrances are usually added in the form of essential oils at about 1% of the total soap weight. For the 9 kg of coconut oil soap described above, about 90 g of essence would be needed. This would be equivalent to about 20 teaspoonsful of citronella oil.
The quantity of dye required for colouring soap is very small; usually, only 0.01-0.03% is needed. For 9 kg of soap this would be about 0.9-2.7 g. The dye should be added as a strong solution, either in oil or water depending on the nature of the dye used. Try dissolving about half a teaspoonful of colour in about 50 ml of water or oil before adding it to the soap. Only edible dyes should be used.
References
ANON. (undated) Soap-making by the cold process. Coconut Development Authority Advisory Leaflet No. 10. Colombo, Sri Lanka: Coconut Development Authority.
DONKOR, P. (1986) Small-scale Soapmaking. London: Intermediate Technology Publications Ltd.
LONSDALE, C. (1989) Straights raw materials for animal feed compounders and farmers. Marlow, UK: Chalcombe publications.
McDONALD, P., EDWARDS, R. A. and GREENHALGH, J. F. D. (1973) Animal nutrition. Edinburgh: Oliver & Boyd.
PALMER-JONES, R. and HALLIDAY, D. (1971) The Small-scale Manufacture of Compound Animal Feed. Report G67. Chatham, UK: Natural Resources Institute. [out of print.]
PARR, W. et al. (1988) The Small-scale Manufacture of Compound Animal Feed. Natural Resources Institute Bulletin 9. Chatham, UK: Natural Resources Institute.
SWEETMAN, A. A. (1992) How to make soap. Food Chain, (5): 19.
Append ices