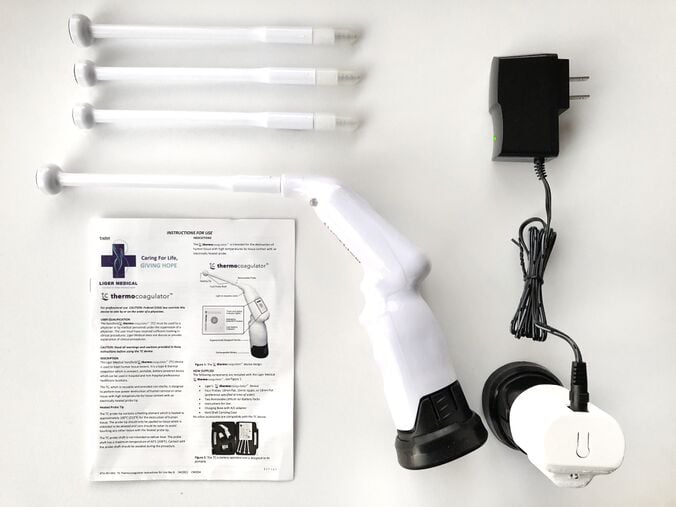
The Liger Medical HTU-110 Thermocoagulator™ (TC) handheld device is used to treat human tissue lesions.[1][2] It is a type B thermal coagulator which is compact, portable, battery powered device which can be used in hospital and non-hospital professional healthcare locations. The following components are included in the Liger Medical HTU-110 Thermocoagulator™ː four probes (19 mm flat, 19 mm nipple, or 16 mm flat; preference specified at time of order), thermocoagulator device, charging base with A/C adapter, two removable lithium ion battery packs, instructions for use, and hard shell carrying case (not shown).
The Liger Medical HTU-110 Thermocoagulator™, which is reusable and provided non-sterile, is designed to perform low-power destruction of human cervical or other tissue with high temperatures by tissue contact with an electrically heated probe tip.
Unlike other devices, the heat-up of the Liger Medical HTU-110 Thermocoagulator™ probe occurs while the probe is in contact with the tissues to help reduce pain symptoms for women during thermal ablation.[3]
Heated Probe Tip[edit | edit source]
**The Liger Medical HTU-110 Thermocoagulator™ probe tip contains a heating element which is heated to approximately 100°C (212°F) for the destruction of human tissue. The probe tip should only be applied to tissue which is intended to be ablated and care should be taken to avoid touching any other tissue with the heated probe tip.
The Liger Medical HTU-110 Thermocoagulator™ probe shaft is not intended to deliver heat. The probe shaft has a maximum temperature of 43°C (109°F). Contact with the probe shaft should be avoided during the procedure.**
Directions for Use[edit | edit source]
For professional use. CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
USER QUALIFICATION[edit | edit source]
The handheld Liger Medical HTU-110 Thermocoagulator™ must be used by a physician or by medical personnel under the supervision of a physician. The user must have received sufficient training in clinical procedures.
CAUTION: Read all warnings and cautions provided in the manufacturer's instructions for use before using the Liger Medical HTU-110 Thermocoagulator™ device.
INDICATIONS[edit | edit source]
The Liger Medical HTU-110 Thermocoagulator™ is intended for the destruction of human tissue with high temperatures by tissue contact with an electrically heated probe.
CONTRAINDICATIONS[edit | edit source]
The user should be familiar with the use of electrical surgical instruments and should take precautions accordingly.
- Using the mobile app, turn ON the Liger Medical HTU-110 Thermocoagulator™ by pressing the ON/OFF button located on the handle once.[1]
- Verify that the green LED is on. The white illumination LED's on the front of the unit will also turn on, and one blue light will flash showing the unit is ready for placement (tip not heated yet).
- When the probe has been placed against the tissue needing treatment, press the ON/OFF button a second time to start the procedure. Four blue timer LEDs will flash sequentially from left to right for a few seconds (~8 seconds) indicating that the probe tip is heating to 100°C.
- When the blue timer LEDs turn solid and a single audible beep is heard, the treatment cycle is running.
- The blue timer LEDs turn off with an audible beep, one at a time, after each 1/4 of the procedure has finished (5 seconds).
- When all four (4) blue timer LEDs are turned off a longer audible beep is heard, the unit is no longer applying heat (~20 seconds). It has commenced its cool down cycle (~10 seconds).
- Once the cool down cycle is complete, the front white LED lights will turn off, and the probe may then be removed from the treatment area.
- If a second treatment area is needed, repeat the above steps before removing the probe.
- Check if the entire TZ has been treated.
- If not, then repeat the procedure so as to treat the entire TZ including the lesion on the ectocervix (1–5 overlapping applications can be used).
Decontamination and Sterilization of the Liger Medical HTU-110 Thermocoagulator™[edit | edit source]
Handle and Battery Cleaning Procedure[edit | edit source]
- Disassemble the Liger Medical HTU-110 Thermocoagulator™ into three separate parts (handle, battery and probe).
- Thoroughly wipe all surfaces of the Liger Medical HTU-110 Thermocoagulator™ handle and battery with a mild cleaning solution (i.e. 70% isopropyl alcohol) or disinfectant and damp cloth.
- The cleaning solution or disinfectant should not be applied directly to the unit.
- Pour/spray the cleaning solution or disinfectant onto a cloth and ensure that the cloth is evenly damp prior to cleaning the unit.
- Do not allow fluids to enter the device.
- Do not sterilize the Liger Medical HTU-110 Thermocoagulator™ handle or battery.
Probe Cleaning Procedure[edit | edit source]
- If possible, initiate instrument cleaning within 30 minutes following use.
- Place silicone cap on probe connector.
- CAUTION: Only use a soft brush or cloth to manually remove impurities; never use abrasive materials as they may damage the probes.
- Perform the final instrument rinse with clean water (i.e. Reverse Osmosis/ De-ionized (RO/DI) water) that does not contribute to device staining or contamination.
Probe Sterilization[edit | edit source]
- Sterilize probes in sterilization trays/pouch and containers. Disposable sterilization packages may also be used
- Place silicone cap on probe connector
- Insert probe(s) into sterilization tray/pouch following sterilizer manufacturer guidelines for appropriate tray/pouch and packaging instructions.
- Ensure that all surfaces will be exposed to the sterilizing agent. Ensure that probes do not contact each other if multiple probes are packaged together.
- Control the water purity dedicated to steam production to prevent damage to the instruments.
- Sterilization temperatures higher than 121°C (250°F) may damage the instruments.
To achieve a sterility assurance level of 10-6 use of the following sterilization parameters is recommended:
Gravity Steam Sterilization Method[edit | edit source]
- Minimum temperature: 121°C (250°F)
- Time: 30 minutes
- Minimum drying time: 30 minutes
Use Case[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 HTU-IFU-002 TC Thermocoagulator Instructions for Use Rev B 04/2021 CN 0354 [product insert]. Liger Medical; 2021.
- ↑ Liger Medical. Thermocoagulator kits: Caring for life, giving hope [Internet]. Liger Medical. Liger Medical; 2021 [cited 2021 Nov 28]. Available from: http://www.ligermedical.com/thermocoagulator-kits/.
- ↑ Wm. Dean Wallace MD, PhD. Personal communication. Liger Medical LLC, October 10, 2021.