
The soil as a planting site[edit | edit source]
The word soil has 2 meanings:
- the earthcrust: the study of the earth's crust = geology or geology
- the planting site for a plant: the study of the soil as a planting site = soil science or pedology
Soil = the upper loose part of the earth's crust up to a depth that is of importance for the plant. The thickness of the soil varies in Belgium between a few cm (high Belgium) to 1-2 m (low and middle Belgium). The section of the soil that is effectively used by the plant = 2m (for trees). Soil cultivation (tillage, fertilization, weeding) needs to be done with the upper 25-30cm of the soil. Consolidaded soil is infertile.
Soil is the medium or substrate through which the plant absorbs their nutrients. This absorption is dependent on many factors such as:
- the amount of water and air in the soil
- the heat of the soil
- Nutrition status and soil acidity
These factors determine whether soil is adequate or not.
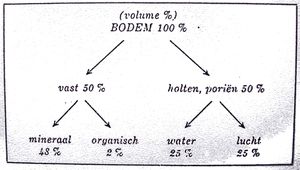
Soil constituents[edit | edit source]
When we look at a clod of earth, we distinguish:
- Solid constituents
- Large and small cavities filled with air or water
Solid constituents[edit | edit source]
a. Mineral constituents Rock particles are created from large rocks due to weathering (wind, water, cold, ...). They are sometimes microscopic (powder-like in appearance). These mineral particles (dead material) form the skeleton of the soil, around which other components group into a complicated complex: the clay-humus complex.
b. Organic constituents These occur in the topsoil and are essentially waste from living organisms (plants and animals). These are further digested to humus in the soil, which is a black sticky substance that gives the soil its dark color. Organic ingredients make life possible in the soil.
c. Living constituents
- Plants: especially plant roots
- Animal organisms: moles, worms, insects
- Micro-organisms: fungi and bacteria
These are important for:
- the conversion into fertilizer
- the formation of humus
Liquid constituents[edit | edit source]
Soil water is not pure but it is a solution of all several compounds of whom the most important are fertilizers. It is present in cavities and pores and unto sticky substances such as humus. It is one of the most important elements in the soil.
Gaseous constituents[edit | edit source]
Soil air has almost the same composition as atmospheric air
| gas | soil air | atmospheric air |
| oxygen | 19,2% | 20% |
| Nitrogen | 80,8% | 78,95% |
| CO2 | 0,2 - 0,7% | 0,03% |
Soil air contains 10 - 20 times more CO2 as atmospheric air. This is caused by the digestion of organic matter and the respiration of the plant roots and soil organisms.
With an aduquate soil, the solid, liquid and gaseous constituents need to be present in a suitable composition.
Soil types[edit | edit source]
Particle size fractions[edit | edit source]
The solid constituents of the soil are more than 90% mineral particles of different sizes, ranging from a pebble to a microscopic particle (clay). The particles are classified according to their particle sizes (called fractions). We use the micron as a measuring unit. A micron is 0,001 mm or 1/1000th of a mm and is indicated by the Greek letter µ (pronounced mu). In the soil, one can distinguish the following fractions using a sieve (soil sieve):

Soil texture[edit | edit source]
Soil texture refers to the sizes of the grains of the soil. It thus refers to what soil type we have (ie sandy, clayish, or loamy soil). The soil can be a mix of these, yet we generalise to sandy, clayish, or loamy soil depending on which of the fractions dominate (eg: 90% sand fractions and 10% clay fractions would be "sandy soil").
These are the soil types.
The soils are divided in Belgium according to their composition.
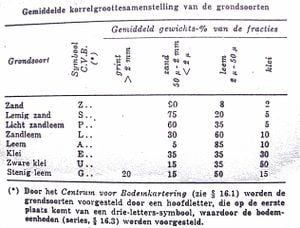
Soils that have more than 30% organic material is called peat soil, those with more than 15% organic material are called humus soils.
Agricultural value[edit | edit source]
In practice, we speak of light and heavy soils, this relates to the manipulatability of the soil. Sandy, loam-sandy, and light sand-loamy soils are easiest to manipulate, these are light soils. Opposed to this are the clayish and loamy soils, which are more difficult to manipulate, these are heavy soils. As for the yield, it's the other way around. The lightest soils are the worst and require a heavy fertilization. Medium light soils such as sand-loamy and loamy soils give the highest yields and have the widest crop choice.
Some cultivations require specific soil types. A light soil supports asparagus (dry loam-sandy soil), Azalea (moderately dry sandy soil), begonia (dry light sand-loamy soil), outdoor vegetables (leek, carrot, cabbage) require light sand-loamy soils, seed onions and cabbage require a loamy to clayish soil.
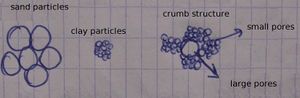
Soil structure[edit | edit source]
By soil structure we mean the way how soil particles lay next to each other; separatly or with fused unto each other to adhesive sets. The soil structure thus refers to the whole of the grain sizes + pores. The alignment of the soil particles next to one another determines the number and the shape of the cavities in the soil or the ratio of soil/cavities. Unlike soil texture, soil structure is affected (beneficially) by tillage operations.
Ratio of soil particles/pores[edit | edit source]
Pores are small openings that are located between the soil grains. The volume they occupy is called the pore volume. They are filled with air and/or water.
- Large pores (>10µ) can not hold any water but drain let it go (draining pores)
- Moderate pores (10 - 0,2µ) hold the water, and also make it available to the plant roots (available water)
- Small pores (0,2µ) hold the water so fiercely that it is no longer absorbable by the plant roots (dead water)
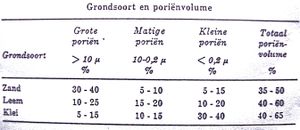
The ratio between the soil particles/pores (air-water) determines the physical fertility of the soil.
Structure of the topsoil[edit | edit source]
Granular and crumb structure.
Grain structure: the soil grains are next to each other without any binding. One can compare them with a pile of grains. It is an inaduquate structure because there are too few holes for a good air-water ratio (pore volume 25%). Only extremely inaduquate soils have this structure.
Crumb structure: the soil grains stick together and form soil crumbs with rounded shapes pile together without any order, similar to breadcrumbs. It is an aduquate structure because the presence of binding substances (humus and clay) contain upto 60% pores so that c a good air-water ratio arises.
The structure of the soil changes regularly (even several times a year) of appearance due to agricultural activities, weather, ... Factors which improve the structure are: introduction of organic matter, tillage, natural phenomena (frost-thaw), crop rotation, workings of the soil organisms, ... Factors that worsen the structure are: precipitation, calcium deficiency, poor tillage, soils that are riden and walked upton (soil compression), ...
Cultivation tips depending on the soil type[edit | edit source]
Some cultivation tips for improving the soil structure depending on the soil type are:
- Less nitrate problems are present with heavier soils (eg soils with more clay, ...)
- clay soils: add lime to coagulate (crumb structure)
- sand soils: add organic substances/humus
- Do not cultivate with rainy weather, and do not cultivate heavily with dry weather (eg hoeing )
Humus in the soil[edit | edit source]
Large quantities of organic waste stream on top of and into the soil, such as plant remains (leaves, roots, stubbles, ...), animal waste (feces). In addition, the farmer also introduces tons of organic manure (stable manure, compost, ...) into the soil. After some time we retrieve nothing of these tons of organic substances, it vanished, consumed by the action of soil organisms (worms) and soil microorganisms (fungi and bacteria). These organisms feed on organic waste that they break it down into water, carbon dioxide (CO2) and nutrients. It leaves only a residual product that cannot decompose further; this is called humus. The conversion process is called the humufication process. 1000 kg of manure leaves only a few grams of humus. Humus is a constantly changing mixture of organic compounds in various stages of decomposition. We distinguish between non-stable or nutrition humus which serves as food for microorganisms (the primary conversion) and stable humus with can be little or no further converted (final conversion). The humification is done by breathing or aerobic bacteria. When the soil contains too little air (due to too much water), the breathing bacteria can not develop and the plant waste is little converted. Peat is then formed (therefore do not introduce stable manure too deep into the soil). Besides air, these bacteria also require nitrogen for their nutrition. Organic material that contains too little nitrogen require the adding of nitrogen for a quick and smooth conversion. The C/N ratio determines the quality of the humus. Organic material with a C/N ratio lower than 30/1 will humificate smoothly. For the introduction of green manure (leguminous plants), the C/N ratio is 15-20/1, so the humus conversion poses no problem. With straw the C/N ratio is 80/1, here the humification requires the adding of nitrogen for a smooth humification process, if this is not done the bacteria will fetch all the required nitrogen from the surrounding soil and a nitrogen-depression could occur. Humus is a very fine colloidal substance = a substance whose molecules are too large to dissolve, but small enough to float in water. Due to the colloidal properties, humus is sticky. With soil analysis we determine not the humus but the carbon content from which we calculate the humus content.
Role of humus:
- With the humification, plant nutrients (N, P, K, ...) are released
- Due to the colloidal properties, humus can hold water and nutrients (clay-humus complex) and it improves the soil structure.
- Due to the dark color, humus accelerates the warming of the soil
- Nutritional humus creates a rich bacterial life and a fertile soil
Water in the soil[edit | edit source]
Importance of water[edit | edit source]

It is necessary for: the plants: the germination of the seeds, the main component of the plant, solvent and transportation of the nutrients, the evaporation (transpiration), maintaining of the sapstream, ... the soil: heat control for the soil (water that evaporates takes heat with it (evaporation)), water influences the soil structure Total evaporation from soil and plant = evapotranspiration. the microorganisms: soil animals and microorganisms need a moist environment.
Types of water in the soil[edit | edit source]
- Soil water: in a well water is present at a certain depth = soil water. The depth to which the groundwater is present is called the the groundwater table. The part below the groundwater table is the groundwater zone; all pores are filled there with water. In temperate countries, the groundwater table rises in the winter and falls in the summer. The cause of this lies in the evapotranspiration. In the summer, the plant and soil evaporation exceeds the rainfall, so the water table drops. In the winter, the evaporation is reduced and the rainfall is greater than the water consumption, so the water table rises.
- Capillary water: water from the groundwater table rises up through the small pores between the soil grains. These fused pores are fine tubes which extend upto the surface. These fine tubes are called capillaries. The water that rises through here is called capillary water. The zone above the groundwater table to where the capillary water rises is called the capillary zone. Its thicknesss depends on the soil type. Water will rise high but only slowly in clay, and will not rise high but fast in sand. Just above the groundwater table, the capillary zone contains much water = closed capillary zone (roots die off). Above it is the open capillary zone, only the fine pores are filled there with water.
- Hanging water: The portion above the capillary zone contains water that, during seepage, kept hanging in the fine pores or got stuck in the colloidal particles. (sandy soils have little or no hanging water, clayish and loamy soils have a lot therof)
- evaporation= soil that evaporates water
- transpiration= plant that evaporates water
Water content of soil[edit | edit source]
Saturation: All pores are filled with water, there remains no room for air. Maximum water content = saturation Field capacity: From a saturated soil layer, water is draint downwards. This takes up to the mement when there is a balance between the suctionforce of the medium and small pores and the force of gravity of the water. The large pores are filled with air, the small and medium are filled with water. The maximum watercontent - drainage water = field capacity Wilting point: The water present at field capacity is only partially absorbable by the plant (available water). Water in the finest pores is so strongly bound that it can not be absorbed by the plants (dead water). A soil with only non-absorbable water = wilting point. Field capacity - available water = wilting point
Cultivation of the soil[edit | edit source]
When all soil untill the capillary water zone/saturated zone is poached, it needs to be left alone for years untill the ground water can once again rise up to the surface via the pores.
Note that the water table of the soil near the coast is unstable concerning surface soil water table (1m variability)
Hydrometers can be used to measure the quantity of water in the soil; if too little water is present, irrigation is needed (this is generally needed only in dry periods). Use a correct planting density and use crop species matched to the local water availability.
Soil acidity[edit | edit source]
Definition of pH and overview[edit | edit source]
The acidity of a solution is determined by the amount of H+ions. In an acid solution the H+ ions are greater than 10-7 gram/liter. In a neutral solution they are equal to 10-7 gram/liter. In an alkaline solution they are smaller than 10-7 gram/liter. Due to the low values, the concentration is often expressed in the pH-value. A solution with a concentration of 10-5 (0,00001) has a pH = 5. Therefore a pH = 5 is 10 times more acidic than a pH = 4.
| PH | Explanation |
| 0-4 | very high acidity |
| 4-5 | highly acid |
| 5-6 | medium acid |
| 6-7 | weakly acid |
| 7-8 | weakly basic |
| 8 | medium basic |
| 8-10 | highly basic |
| 14 | very highly basic |
between 5-8 PH is thus acceptable
the soil PH can shift by 0,5-1 according to the time of year
PH-H2O (free hydrogen-ions) -->clay-humuscomplex -->current acidity/basicity
PH-KCL: (free hydrogen-ions +clay-humuscomplex-ions) -->total acidity/basicity
The difference between the 2 is the degree of exchange in acidity
Ideal PH-values per soil type are:
- for sandy soil: 5,4 PH
- for loamy soil: 7 PH
Depending on the crop to cultivate, more or less acid soil needs to be used
increasing/decreasing soil PH -->extensive task, requires several years
pH in soil science[edit | edit source]
We find the acidity of the soil by measuring the H+ ions using a liquid that had sufficient time in contact with the soil. pH-water or the current acidity-level: when we dissolve the soil in distilled water and then measure the pH thereof, we measure the free H+ ions that are in the solution. pH-KCl or the total acidity level: when we dissolve the soil in a KCl-solution, we measure not only the free H+ ions, but also those ions who got stuck into the soilcolloïds (clay-humus complex). The difference between pH-KCl and pH-water =exchange acidity level. pH-KCl is always 0,5 to 1 pH unit lower. For professional purposes, pH-KCl is increasingly measured as pH-water fluctuates somewhat according to the seasons. (towards summer, the pH drops)
How to measure the pH
- Litmus paper
- blue in alkaline medium (pH 7)
- red in acid medium (pH 7)
- With electronic pH-meter = introduce electrode in solution
Soil type and pH[edit | edit source]
For all soil types, we must be state that the best pH-water is between 6 and 8. As the soil gets lighter, the pH needs to be lower.
| pH/H2O | pH/H2O | |
| sandy soil | 5,7 - 6,3 | average at about 6 |
| sand-loamy soil | 6,3 - 7,0 | average at about 6,5 |
| loamy soil | 7,0 - 7,5 | average at about 7,0 |
| clayish soil | 7,5 - 8,0 | average at about 7,5 |
Cultivation and pH Most vegetables prefer a weakly acid to neutral soil pH = 6 to 7. Typical heathland plants such as Erica and Azaleas prefer a pH = 4-5. At a pH below 5,5 diseases start to occur because the absorption and conversion of the nutrients is disturbed or even impossible.
A high pH is rare, a low pH is frequently encountered in Belgium.
Changing the PH[edit | edit source]
Besides the use of lime, scattering seaweed calcium increases the PH, leaves dry more easily
Substrates[edit | edit source]
Substrates are mixes of naturally occuring soil types, or artificial "soils".
Organic substrates[edit | edit source]
Inert substrates[edit | edit source]
- rock wool (vulcanic origin)
- polyurethaan
- pumice
The buffer-capacity of organic substrates is much higher than of inert substrates.
Plant nutrients[edit | edit source]
Carbon dioxide (CO2) and water (H2O) are the main plant fertilisers; they supply the elements C, H and O, essential building blocks of all organic parts of the plant. In addition, the plant has a number of necessary elements required for several organic compounds.
- Main elements of macro-elements: N, P, K, S, Ca, Mg of which the plant needs relatively large quantities.
- Trace elements or micro-elements: Mn, Cu, B, Mo, Zn, Fe of which the plant needs only very small quantities in comparison to the main elements (up to 1000 times less)
- Non-essential elements: of which the plants can be develop themselves even in their absence: Na, Cl, Al, Si
In practice, fertilision is mostly done using N, P, K. The soil in Belgium (for example) contains sufficient amounts of S, so that adding this is not necessary. Ca is supplied together with different N- and P-fertilizers. Lime fertilizers are not supplied because of Ca-needs but to improve the pH and the soil structure. Trace elements are often found in small amounts in the administered fertilizers.
All nutrients except for CO2, are generally absorbed by the plant from the soil. Plant nutrition comes in several forms:
- dissolved in soil water as ions: directly absorbable by the plant
- Adsorbed unto the clay-humus complex: clay and humus particles are electrically negatively charged. Thus they attract positively charged particles (H+, K+, Na+, NH4+, Ca2+, Mg2+, ...) and hold these. Trough exchange with positive ions from the soil water, these are released again and can be absorbed through the plant.
- Mineral reserve: solid constituents of the soil consist of minerals (carbonates, phosphates, silicates, quartz). These include Na, K, Ca, Mg, Fe which are gradually released by the weathering of the rocks.
- Organic reserves: the remains of dead plants and animals that are not directly absorbable. They are gradually made available to the plant by mineralization. The total reserve on plant nutrition is often enormous, only a small portion of this is absorbable by the plant.

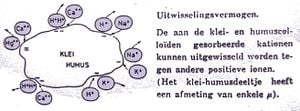
Soil analysis[edit | edit source]
A soil analysis includes the determination of:
- the soil type
- the level of acidity in PH-KCl
- the level of humus in carbon %
- the absorbable level of P, K, Mg, Ca and Na for the plant
On request, additional determinations can be implemented, such as the determination of specific trace elements. The levels of nutrients are expressed in mg pure element per 100g air-dry soil. The analysis results can be compared with a target result. The target result is specific for each lot and takes into account the soil type, the level of carbon and probing depth. The level of fertilisation is categorised into 7 rating classes.
Soil analysis examples[edit | edit source]
Example 1 of a soil analysis (Soil analysis service of Belgium):
Plot details B.D. Serial: T0007111 Nr. of the probing: JR21 (4) lot name: Garden
Analysis results and assessment:
| Determination | Determination result | Target figure* | Assessment |
| Soil type | 10 | ||
| Coarse sand | |||
| Acidity: pH-KCl | 7,3 | 5,4 | High. Do not lime. |
| Humus: carbon | 2,2 | 2,3 | Normal. |
| Phosphorus (P) | 65 | 21 | Very High. Do not administer phosphorus for the next years. |
| Potassium (potash) (K) | 42 | 27 | High. At most, only administer provisional light potash manures |
| Magnesium (Mg) | 28 | 8 | High. Magnesium fertilisation is unnecessary for the moment. |
| Calcium (Ca) | 535 | 103 | High |
- Target figures are individual per plot. They are dependant on the texture (soil) and the humus content (volume/weight) for a given soil. With numbers off the target figures, the ratio can be more important than the level itself.
Fertilisation advise: in kg/are (1 are = 100m²) for a vegetable garden
Liming: 0.0 kg acid binding value per 100m² Nitrogen: Following nitrogen fertilization is to be considered as an average for this plot with the user-specified crop (all per 100m²).
For coal, leek, tomatoes, celery, spinach, etc: approx. 1.5 kg of nitrogen. This corresponds (in commercial fertilizer) to:
- Approximately 5.6 kg ammonia nitrate 27% N
- Approximately 15.0 kg of nitrogen fertilizer and 10% N
- Approximately 7.3 kg ammonium sulphate 20.5% N
For potatoes, onions, endive, cucumbers, salsify, lettuce: approximately 1.2 kg of nitrogen. This corresponds (in commercial fertilizer) to:
- Approximately 4.4 kg ammonia nitrate 27% N
- Approximately 12.0 kg of nitrogen fertilizer and 10% N
- Approximately 5.9 kg ammonium sulphate 20.5% N
For strawberries, shallots, sprouts, lettuce, carrots, approximately 0.8 kg of nitrogen. This corresponds (in commercial fertilizer) to:
- Approximately 3.0 kg ammonia nitrate 27% N
- Approximately 8.0 kg of nitrogen fertilizer and 10% N
- Approximately 3.9 kg ammonium sulphate 20.5% N
Additional determinations:
Analysis results (in mg/kg dry soil = ppm)
| Copper (Cu) | 10.42 ppm | normal levels |
| Lead (Pb) | 37.52 ppm | normal levels |
| Zinc (Zn) | 64.43 ppm | normal levels |
| Cadmium (Cd) | 0.33 ppm | normal levels |
| Cobalt (Co) | upto 2.56 ppm | normal levels |
| Boron (B) | 0.90 ppm | relatively high levels |
Conclusion: The boron level is fairly high, we recommend to not give any boron fertilizer. All other levels are favorable normal levels. As far as the analyses allow to determine, the cultivation of vegetables can be done without any health risk.
| PH (KCl) | Humus (%C) | P, K, Mg, Ca, Na, B, Cu, Co |
| 1: strong acid | 1: very low | 1: very low |
| 2: Low | 2: Low | 2: Low |
| 3: rather low | 3: rather low | 3: relatively low |
| 4: favorable | 4: normal | 4: normal |
| 5: fairly high | 5: fairly high | 5: quite high |
| 6: High | 6: High | 6: High |
| 7: very high | 7: boggy | 7: very high |
Example 2 of a soil analysis (Soil Service of Belgium):
Plot Details Serial B.D.: SO206010 Nr. of the probing: 133 (4) Lot name: Van Ygem
Analysis results and assessment:
| Determination | Determination result | Target figure* | Assessment |
| Soil type | 35 | ||
| Light loam | |||
| Acidity: pH-KCl | 6,4 | 6,4 - 6,9 | Favorable |
| Humus: carbon | 1,8 | 1,2 - 1,6 | Normal. |
| Phosphorus (P) | 37 | 13 - 21 | High. |
| Potassium (potash) (K) | 42 | 16 - 23 | High. |
| Magnesium (Mg) | 14 | 10 - 17 | Normal. |
| Calcium (Ca) | 167 | 183 - 403 | Fairly low. |
| Sodium (Na) | 4,6 | 3,5 - 6,9 | Normal |
| Boron (B) | |||
| -- |
Liming advise (total dosage): 1300 kg acid binding value (abv) per hectare (as maintenance liming)
Fertilization advice: in kg/ha for:
| first crop | second crop | third crop | |
| (7/89, leek) | (5/90, parsley) | (4/91, spinach) | |
| lime | 1300 kg a.b.v. | 0 kg a.b.v. | 0 kg z.b.w. |
| Nitrogen | 200 kg N | 150 kg N | 150 kg N |
| Phosphorus | 40 kg P2O5 | 40 kg P2O5 | 80 kg P2O5 |
| Potassium | 90 kg K2O | 40 kg K2O | 190 kg K2O |
| Magnesium | 130 kg MgO | 130 kg MgO | 80 kg MgO |
| Sodium | 0 kg Na2O | 0 kg Na2O | 0 kg Na 2O |
| Boron | -- | -- | -- |
Importance of the nutritional elements[edit | edit source]
Main elements[edit | edit source]
Nitrogen (N): Nitrogen is of great importance in the plant for the formation of proteins, chlorophyll and other organic compounds. It promotes the development of leafs and stem. When there is a nitrogen deficiency, the above-ground parts stay small and branch little. Too little leaves develop which also stay small. Leave stems remain short and are pointed steeply upwards. The plant gets a bright green color (chlorophyll deficiency). Excess nitrogen can be recognized due to a strong vegetative growth with long dark green leaves and soft shoots. Sometimes bloom delays occur and there is a reduced fertility. Woody stems ripen slowly and are therefore susceptible to frost. There is also a greater risk of infestation by aphids and mildew fungi. Too much N also reduces the absorption of potassium, so that potassiumdeficiency occurs.
Phosphorus (P): Phosphorus is necessary for the formation of core proteins and plays a role in the assimilation and respiration by the plant. It has a favorable influence on the development of the root system and promotes the ripening of the crop. It increases the sugar and starch content in roots and tubers. A lack of phosphorus is most recognizable in a young crop. When there is a deficiency, the leaves turn purple and reddish-brown at the bottom of the leaf. Poor rooting and low yields also accompany phosphorus deficiency. Plants remain small and delicate. Flowers are also smaller than normal.
Potassium (K): is important in the metabolic processes in the plant. It therefore has a beneficial effect on the yield and the quality of the crops. It promotes the production of carbohydrates. It reduces the sensitivity to drought and frost. When there is a potassium deficiency, the leaves keep behind in growth and are puckered or curled. The color of the leaves are darker than normal. The leaf edges are colored yellow and also withered. Potassium deficiency leads to poor maturation of woody plants in the autumn, making them sensitive to frost.
Sulfur (S): Both sulfur deficiency and excess sulfur give no specific symptoms. The signs of deficiency have some similarity to N-deficiency.
Magnesium (Mg): Magnesium is important as a building element for chlorophyll. With a magnesium deficiency, the leaves retain a yellow discoloration along the leaf edges or between the leaf veins. Later-on, a brown discoloration occurs. Along the leaf veins, the leaves remain green. The uptake of Mg occurs mainly in cold wet summers. At a soil pH lower than 5, there is almost no more absorption of Mg.
Calcium (Ca): Calcium is important for the plant to neutralize acids. It is a component of the cell walls. Calcium deficiency is little observed. With some fruits (eg tomato), the deficiency of calcium is expressed by nose rotting. Ca is of more importance to the soil.
Trace elements[edit | edit source]
Copper (Cu): promotes the formation of chlorophyll and is part of some enzymes. Deficiency symptoms are mostly found at sandy soils that are poor in humus content. One sees a poor development of growing tissue sections. Leaves are sometimes chlorotic and/or necrotic and curled or twisted.
Zinc (Zn): Zinc deficiency can occur in poor soils and soils with a high pH. Usually there is a compact growth and chlorotic spots in older leaves. A fertilisation with an organic manure is often sufficient to eliminate the problems.
Boron (B): Boron plays a role in the transport of carbohydrates in the plant. Boron deficiency often leads to the death of the growpoints and young shoots, after which they start to decay (affects cell division). Borondeficiency occurs mainly on light sandy soils, especially during dry periods. At a high pH the risk of deficiency is also increased.
Molybdenum (Mo): Molybdenum deficiency leads to a keeping behind in growth, leaves that become pale and wither eventually. Symptoms often appear in the middle and oldest leaves. They are yellow to yellowish-green and the leaves curl. Mo deficiency usually occurs on soils with a low pH, the remedy will mainly consist of bringing up the pH.
Manganese (Mn): Manganese is necessary for the formation of chlorophyll and plays a role in the physiology of the plant. With manganese deficiency, the young leaves become yellow spotted, with veins that remain green. With manganese deficiency, the plant produces too little carbohydrates which renders the plant limp and ragged due to a lack of formed cellulose.
Iron (Fe): Iron is needed in the formation of the green color of the leaves. Iron deficiency expresses itself as a yellowing (chlorosis) in the young leaves, where the veins remain green. With serious deficiency, the growth comes to a halt and white coloration of the leaves occur.
Deficiencies
| Cause | P | K | Mg | Ca | B | Fe | Mn | Zn | Cu | Mo |
| low pH | x | x | x | |||||||
| high pH | x | x | x | x | x | |||||
| too dry | x | x | x | x | ||||||
| too wet | x | x | ||||||||
| light soil | x | x | ||||||||
| cold ground | x | x | x | |||||||
| too high N | x | |||||||||
| too high P | x | x | x | x | ||||||
| too high K | x | x | x | |||||||
| too high Fe | x | x |
Determination of deficiencies[edit | edit source]
A. Symptoms firstly occuring on older leaves
- Abnormally formed leaves, reduced growth, no necrosis
- Yellow, monocotyledons: V-shaped yellowing starting at the top: N
- Purple or very dark green coloring of the leaves: P
- Necrosis and/or chlorosis
- Brown bronze necrosis starting on the leaf edge or top: K
- Between-vein chlorosis: Mg
B. Symptoms firstly occuring on younger leaves
- Dead end bud; especially with dicotyls: B
- Weakened stem, angled at the top: tomato (nose rotting), apple (spots): Ca
- Shortening of the internodes: Zn
- Young leaves wilted, poor flowering and setting of seeds: Cu
- Between-vein chlorosis: Fe
C. Symptoms throughout the entire plant
- Legumes and coal (clamping heart): Mo
Source[edit | edit source]
This article is made using information from the coursebook:
Cultivation techniques
(Part 1: Soil science)
Course from: 1st year of Herbalism
Course year: 2005 - 2006
Education center: Syntra, Asse-establisment
Teacher: Leo Van Crombrugge
