Contact Precipitation
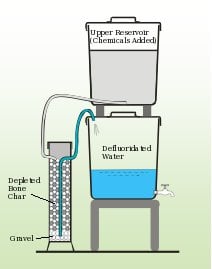
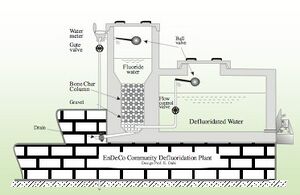
A more recent development in the usage of bone char in defluoridation is the method of contact precipitation. This method is based on the addition of chemicals to water before running the water through depleted bone char filter media. By adding calcium chloride (CaCl2) and monosodium phosphate (NaH2PO4) to the water the precipitates of fluorapatite and calcium fluoride are formed and removed from the water.[1]
CaCL2 2H2O => Ca2+ + 2Cl-+2H2O
NaH2PO4H2O => PO43- + Na+ +2H+ +H2O
Ca2+ + 2F- => CaF2 (s)
10Ca2+ + 6PO4- + 2F- => Ca10(PO4)6F2
At this point there is not a thorough understanding of how contact precipitation works. It has been noted that the addition of calcium to fluoride rich water generally does not lead to significant precipitation of CaF unless it is in the presence of saturated bone char which catalyses the process. The addition of the calcium and phosphate may also help to coat the surface in a new layer of hydroxyapatite which may be able to add effectiveness to the depleted char.[2] It is also speculated that the addition of calcium to the depleted bone char helps to repair some of the hydroxyapatite structure destroyed in the charring process. Filters to operate for contact precipitation are designed similarly to normal bone char filters except that they have a larger reservoir for the input of raw water so that the chemicals can be added before passing through the depleted char. A effluent reservoir or bed is then added to be able to collect the water without the precipitate. It should be noted in the design and construction of this filter that residence times of 20-30 minutes have been shown to be most effective.[1] Some sketches of potential filter setups can be seen below. This method was developed in East Africa and has been successfully tested and implemented at a village and school level in Arusha, Tanzania successfully.[1] Though not yet widely used on a household level, it is believed to be appropriate for household usage because it is considered to be reliable, have high fluoride removal efficiency, be cheap, and no real risks associated with improper chemical dosage. The primary barrier to the adoption of contact precipitation methods are the efforts required by the users to apply the dosages of the chemicals (though considerably easier than the Nalgonda technique) and the availability of said chemicals.[2]
Sample Calculations to determine Design Criteria associated with Contacte Precipitation. CaCL2 is assumed to contain 27% Calcium and NaH2PO4 65% Phosphate
| Given Parameters: | Unit | Domestic | School/Market | |
|---|---|---|---|---|
| D | Daily personal water demand | L/(c d) | 3 | 0.5 |
| N | Number of users | p | 6 | 500 |
| Fi | Raw water fluoride conc. | mg/L | 10 | 10 |
| Ft | Treated water average fluoride conc. | mg/L | 0.4 | 0.4 |
| E | Medium porosity | - | 0.56 | 0.56 |
| s | Medium Bulk Density | kg/L | 0.83 | 0.83 |
| v | Filtration velocity | m/h | 0.5 | 0.5 |
| tc | Contact time (=HcxE/v) | h | 0.3 | 0.3 |
| tf | Filtration time (=Q/(v x pi x (Ø/2)2))) | h | 4 | 4 |
| VRRW/O | Volume ration raw water/daily water treated | - | 1.1 | 1.2 |
| VRBC/O | Volume ration raw water/daily water treated | - | 0.3 | 0.3 |
| VRCW/O | Volume bone char medium/daily water treated | - | 1.1 | 2 |
| MRCC/F | Mass ratio calcium chloride/daily fluoride loading | - | 30 | 30 |
| MRMSP/F | Mass ratio MSP/daily fluoride loading | - | 15 | 155 |
| Derived Parameters | ||||
| Q= D x N | Daily water treatment | L/d | 18 | 250 |
| FT=QxFi/1000 | Total daily fluoride loading (removal) | g/d | 0.18 | 2.5 |
| ØBC= 2(Q/tF x v x pi)0.5) | Diameter of contact bed | cm | 11 | 40 |
| HBC= tC x v/E | Height of contact bed medium only | cm | 27 | 40 |
| Mcc=FT x WRCC/F | Total daily dosage of CC | g/d | 5 | 75 |
| MMSP= FT x MRMSP/F | Total daily dosage of MSP | g/d | 3 | 40 |
| VRW=Q x VRCW/O | Volume of raw water bucket/column | L | 20 | 300 |
| VBC= Q x VRBC/O | Volume of contact bed medium | L | 2.4 | 33.5 |
| MBC= VBC x s | Mass of contact bed medium | kg | 2 | 30 |
| VCW= Q x VRCW/O | Volume of clean water bucket/tank | L | 20 | 500 |
| Corresponding Parameters | ||||
| WRW= (VRW)1/3 | Width of raw water column | cm | - | 65 |
| LRW= WRW | Length of raw water column | cm | - | 65 |
| HRW= VRW/(WRW x LRW) | Height of raw water column | cm | - | 70 |
| ØCB= ØBC | Diameter of contact bed compartment | cm | - | 40 |
| HCB= HCW | Height of contact bed compartment | cm | - | 65 |
| WCW= WRW | Width of clean water tank | cm | - | 65 |
| HCW= HCB | Height of clean water tank | cm | - | 65 |
| LCW= VCW/(BCW x HCW) | Length of clean water tank | cm | - | 120 |
Soils, Clays, and Minerals[edit | edit source]
Various soil and clay types have long been used for water treatment, and there are records of ancient Egyptians using clay to treat turbid water since ancient times.[1]

A number of soils have been used in defluoridation including: Magnesite, apophyllite, natrolite, stilbite, clinoptilolite, gibbsite, goethite, kaolinite, halloysite, bentonite, vermiculite, zeolite, serpentine, alkaline soil, acidic clay, kaolinitic clay, China clay, aiken soil, Fuller's earth, diatomaceous earth, lateritic clay, and ando soil.[1] All of these soils, most composed chiefly of oxygen, silicon, and aluminum, have lattice hydroxyl-groups which are exchanged for fluoride.[1] Typically, these materials are calcined, acid washed, or air dried before use.[1] Clays, which are probably some of the more utilized materials listed, are typically calcined at temperatures of 500-700oC with has been shown to be the optimal temperature for calcining to encourage fluoride binding in the material. One study has proposed that though calcining may improve the fluoride uptake abilities of many clays, it is not necessary for fluoride removal. However, calcining is still recommended for the purpose of sterilizing the filter media.[3] One must also be careful as calcining at temperatures of over 800oC have shown to reduce fluoride removal potential or even result in increasing the fluoride content of water. In addition to concerns of sterilization, the usage of soils and clays also requires careful selection of materials, as some soils may actually increase the fluoride content of water when used as a filter media.[4] Though yet to be proven as universally true, there is reason to believe that generally clays and soils from highlands may be more effective in fluoride removal as they often have lower fluoride content..[4] These defluridation filter media are typically crushed and placed into an upflow filter column or bucket, in order to allow for the settling of suspended solids within the filter bed.[1]
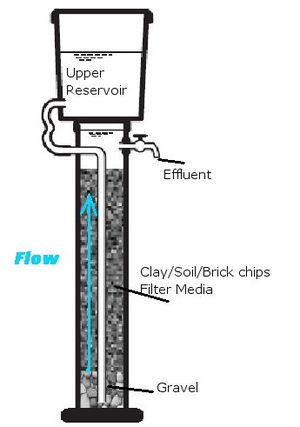
The chief advantage of using clays and soils is that it is locally available and cheap. Though there is significant variability in the fluoride uptake capacity of these materials, they generally have far lower capacities and treat water far too slowly compared to other adsorbtion methods such as bone char or activated alumina.[5][3]
One exception to this general is the use of Magnesia (MgO) in defluoridation. Magnesia is created by calcining magnesite (MgCO3) and crushing it into small pieces to be used as a filter media.[6] It fosters an ion exchange process and has been shown to have an extremely high removal capacity of over 30 mg F/g..[7]It has the potential to be an especially promising technology in Tanzania where many magnesite mines have been established.[7] Though it has been discussed as a potential solution to defluoridation since the 1930s it has never been successfully implemented.[7] This is largely because the chemical reactions associated with the ion exchange are self-regulating in regards to pH, automatically moving the pH of the solution to its optimal point of 10.5-11.[8]Thus the use of magnesia in defluoridation requires additionally treatment afterwards to lower the pH to an acceptable drinking level.[9]
Clay filters have been used with some success in a number of regions, most notably Sri Lanka.[1]
Below is a sample calculation of the design parameters associated with the construction of a clay filter. Because there is significant variability in constants associated with this filter media, it is assumed that a bucket filter uses clay powder with a capacity of 0.03 mg/g and bulk density of 1.5 kg/L, and a column filter has clay brick grains of 8-16mm, with a capaciry of 0.1mg/g and bulk density of 1.3 kg/L
| Given Parameters | Unit | Bucket Type | Column Type | |
|---|---|---|---|---|
| D | Daily personal water demand | L/(c d) | 3 | 3 |
| N | Number of users | p | 6 | 6 |
| OP | Operation Period | days | 1 | 180 |
| Lo | Operational sorption capacity | g/kg | 0.03 | 0.1 |
| s | Bulk Density of Medium (Powder & Chips) | kg/L | 1.05 | 0.086 |
| Fi | Raw water fluoride conc/ | mg/L | 3 | 3 |
| Ft | Treated water average fluoride conc. | mg/L | 1 | 1 |
| VRSW/M | Volume ratio supernatant water / medium | - | - | 1/5 |
| VRAF/M | Volume ratio after-filter water / medium | - | - | 1/2 |
| VRS/Q | Volume ratio of sludge / water demand | - | 1/10 | - |
| VRVS/Q | Volume ratio vacant space for mix./ water demand | - | 1/15 | - |
| Derived Parameters | ||||
| Q=DxN | Daily water treatment | L/d | 18 | 18 |
| Vs= QxVRS/Q | Volume of Residual Sludge | L | 2 | - |
| VT= OPxQ+Vs | Total volume of water treated in a filter period | L | 20 | 3200 |
| FT=VTx(Fi-Ft)/1000 | Total fluoride removal during a period | g | 0.04 | 6 |
| M=FT/Lo | Amount of medium required for removal | kg | 1.3 | 65 |
| VM=M/s | Volume of the medium in the filter | L | 0.9 | 50 |
| BV=VT/VM | Number of bed volumes treated in a filter period | - | - | 45 |
| VSW=VMxVRSW/M | Volume Capacity of supernatant water | L | - | 15 |
| VAF=VMxVRAF/M | Volume of after-filter arrangement | L | - | 40 |
| VVS=QxVRVS/Q | Volume capacity of vacant space in bucket | L | 1.2 | 0 |
| VB=Q+VS+VVS;VF=VM+VSW+VAF | Total Volume of bucket/filter | L | 40 | 130 |
| Corresponding dimensions | ||||
| Ø | Filter diameter (selected as available) | cm | 35 | 40 |
| H=VB/(pi x (Ø/2)2 or VF/(pi x(Ø/2)2) | Total height of the bucket/filter | cm | 40 | 100 |
Nalgonda Technique[edit | edit source]
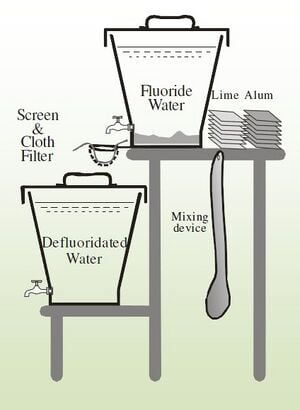
The Nalgonda technique is a means of fluoride removal that depends on the flocculation, sedimentation, and filtration of fluoride with the addition of aluminum sulfate and lime. This technique was developed by the National Environmental Engineering Research Institute in India in 1975 in response to fluorosis concerns.[10] Aluminum sulfate (Al2(SO4)318H2O) is added to the water to acts as a flocculent. Though aluminum sulfate is commonly used in general water treatment as a flocculent, the amounts used in defluoridatoin are much higher (150 mg/mgF or 1000mg/L or 20 times normal).[11] As is typical with flocculation processes, the water must be thoroughly stirred to ensure dispersal of the flocculating agent.[1] Because the reaction results in an excess of H+ ions, Lime (Ca(OH)2) is added to the water during the process to help maintain a neutral pH and hasten the settling of the sediment. The amount of lime added is typically 5% (by mass) of the aluminum sulfate added though some sources say significantly more (20-50% of alum by mass) should be added.[1] The chemical processes, though admittedly are not fully understood,[11] can be seen below:
Al2(SO4)318H2O => 2Al + 3 SO4 + 18H2O
2AL + 6H2O => 2Al(OH)3 + 6H+
F- + Al(OH)3 => Al-F Complex +undefined product
6Ca(OH)2 + 12H+ => 6Ca2+ + 12H2O
Additionally, some of the fluoride is able to form precipitate with calcium.[12]Ca(OH)2 + 2F- => CaF2 +2OH-
In order to determine the amount of aluminum sulfate that should be added the following equation can be used.[1]
A=((Fr-Ft)xV)/(mxFt1/n)
Fr= The raw water fluoride concentration (mg/l)
Ft= The residual water fluoride concentration (mg/l)
V= The Volume of water treated (l)
m= The sorption capacity constant
n= The sorption intensity constant
A= The amount of Alum to be added (g)
(Under near neutral pH and target concentrations of 1.5 mg/L m=6 and n=1.33)[1]
After waiting for the sediment to settle, filtration is then needed to ensure that none of the created sediment is drank.[1] The actual amounts of aluminum and lime to be added can vary greatly depending on the initial alkalinity, fluoride concentration, and the quality of lime.[11] The amount of aluminum sulfate added must be carefully monitored as both left over aluminum can cause significant health problems including neurological, cardiovascular, and respiratory problems among others and must be kept under 0.2mg/l.[12] Although less serious, left over sulfates must also be monitored as they can cause poor tastes, and must be kept under under 400mg/L which means that no more than 800mg/l of aluminum sulfate should should initially be added to the water.
One advantage of the Nalgonda technique it that is easily scalable and can be used from a household system of buckets to and large scale plant.
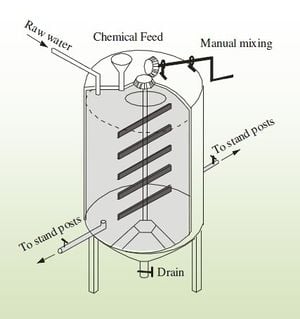
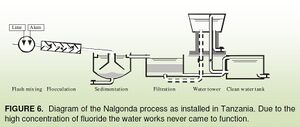
As with other defluoridation systems, the Nalgonda technique is often used as a POU treatment, and has been successfully used in many developing nations including, India, China, and Tanzania. People are taught to add the correct amounts of alum and lime based on the fluoride and pH of the raw water being used and to quickly stir in the added chemicals for 1 minute, continue stirring slowly for 5 minutes, and then let it settle for 1 hour.[1]
The Nalgonda Technique has not been embraced on a large scale in the developing world. There are a number of serious drawbacks to the method. Though often considered to be a cheaper defluoridation method available, it is not necessarily cheaper everywhere, particularly where necessary materials are not readily available, such as East Africa. Additionally there are limitations chemically as the Nalgonda Technique has been shown to not be sufficient for the treatment of water with a fluoride concentration greater than 10.0 mg/L. Also the pH is very difficult to regulate with the addition of lime,[11] and sludge created needs to be properly disposed of.[1] Finally, the Nalgonda technique is more time intensive and requires more diligence than other defluoridation options and this is possibly the largest reason why it has yet to be embraced.
Other Defluoridation Options
Reverse Osmosis
Reverse osmosis is a technology that has been used more successfully in the developed world than the developing world. This process is achieved by applying high pressure to water against a semipermeable membrane that is capable of rejecting undesired ions from passing through.[12] A variation of this process is known as electrodialysis that relies on DC potential to remove specific ions. In fact, reverse osmosis can be used to remove a variety of undesired quantities from the water depending on the nature of the membrane used.[12] Being a purely physical process, it eliminates many of the problems seen with other defluoridation techniques, like pH balancing and the need for regeneration.[12] Reverse Osmosis has been shown to successfully treat water with fluoride concentrations up to 12 mg/L. Unfortunately, reverse osmosis has not been successfully implemented in the developing world for a number of reasons. The primary being that it is a very costly defluoridation option. Additionally, reverse osmosis requires much electrical power to operate. Also, 20-40% of water is lost in this treatment process, possibly much more. Technological improvements in materials and larger scale operations may someday make this technology affordable to more people in need of defluoridation technology.[12]
Magnesium Oxide
Testing of Defluoridating Filter Using MgO-CaO-CaCl2 For Use in Rural Rajasthan, Southern Brazilian Journal of Chemistry, Margandan, Agrawal, R; K; Singh, K; Acharya, R; Sharma, S; Qanungo, Kushal,Vol. 21, No. 21, 2013, p 79-95. http://web.archive.org/web/20160331192736/http://sbjchem.he.com.br/jornal/revista2013.pdf
Field Testing of a Magnesium Oxide-Lime-Calcium Chloride Hydrochloric Acid Based filter, Margandan, Agrawal, R; K; Singh, K; Acharya, R; Sharma, S; Qanungo, Kushal, Ovidius University Annals of Chemistry Volume 24, Number 1, p 43-50, 2013. http://anale-chimie.univ-ovidius.ro/anale-chimie/chemistry/2013-1/pdf/9_karunanithi.pdf
Synthetic Materials
There are a number of strong base anion exchangers that are able to perform an ion exchange process to remove fluoride from water, typically exchanging for chloride ions.[12] Many of these are not specifically designed for use in removing fluoride, but rather all anions. Thus because they are not specifically designed for removing fluoride, their actually fluoride removal capacities are relatively low.[13] Though able to be regenerated with the use of chloride salts,[12] they are not necessarily the most economical and available in areas where they are not produced, and thus, at this point, are not able to really be considered an alternative in the developing world.
Biological Options
Though relatively unknown, some success has been seen in using biological materials such as strains of fungi, bacteria, and algae.[14] The mechanism of fluoride uptake with these methods are largely not understood.
Additionally, studies have been performed by creating activated carbon from water Hyacinths (Eichhornia crassipes).[12] By charring these plants at 600oC fluoride capacities as high as 4.4 mg/g of carbon were seen.[12]
Solar distillation
Solar distillation can also be used to remove fluoride from drinking water and F concentration in distilled water will be within the permissible limit of 1.5 mg/L. However, this process mainly weather depended.[15]
Alternatives to Defluoridation[edit | edit source]
Of course there are a number of ways to help reduce the occurrences of fluorosis other than just treating water. All of the water resource options available should be assessed economically, as it may be more affordable to find and use a different water source. (Often times a water source should just be abandoned rather than treated, especially if it has exceptionally high levels of fluoride.) Rainwater often can provide a fluoride free water source,[12][16] though this isn't universally agreed upon. Sometimes if multiple water sources are available, the source with lower fluoride concentrations can be used to dilute the fluoride concentration of another.
Additionally, diet can play a significant role in the effects fluorosis has on the body. A diet that is high in protein, vitamin C, calcium can help reduce the effects of fluorosis.[17] That being said, milk consumption should be encouraged.[18] On the other side, general malnourishment generally exacerbates the harmful effects of fluoride[17]and a diet that is high in silicon, a mineral critical in bone mineralization, also has been shown to make the effects of fluorosis worse.[17]
Evaluation of Defluoridation Treatment Types[edit | edit source]
There are a number of different defluoridation options, none of which emerge as being more appropriate in all situations. Thus many factors must be taken into account when deciding on what defluoridation option to use. A number of questions should be asked when selecting a technology, including:
Economic Factors
What is affordable to those using this defluoridation method?
Are the proper supply chains in place to ensure that people will always have access to the materials needed for this defluoridation method?
Social Factors
Is this defluoridation methodology simple and easy to use?
Does this technology require any special know-how, skills, or tools?
Are people willing to use this defluoridation method?
Is the water from this treatment method acceptable to drink?
Technical Factors
Does this techology have the fluoride removal capacity necessary for the region's level of fluoride?
Is this technology selective for fluoride/does the water have characteristics that can interfere with the uptake of fluoride?
Does this technology require frequent replacement,recharge, or repair?
Environmental Factors
Does this defluoridation technology have any undesirable by products?
What resources does this technology consume?
An Overall Comparison of Fluoride Removal Techniques for Use in the Developing World (+=PRO, -=CON)
| Activated Alumina | Bone Char | Calcined Clay | Contact Precip. | Nalgonda | |
|---|---|---|---|---|---|
| No daily dosage of chemicals (i.e. no daily working load) | + | + | + | - | - |
| Dosage designed for Fluoride Conc. independent of unit or plant | - | - | - | + | + |
| No risk of false treatment due to breakpoint | - | - | - | + | + |
| Removal capacity of medium is independent of Fluoride conc. | - | - | - | + | - |
| No regeneration or renewal of medium is required | - | - | - | + | + |
| High removal efficiency can be ensured | + | + | - - | + | - |
| Easy to construct, even by users | + | + | + | + | + + |
| Construction materials are cheap and widely available | + | + | + | + | + + |
| Can be sized for one or several families or e.g. a school | + | + | - | + + | + |
| No risk of medium/chemicals unacceptability | + | - | - | - / + | + |
| No risk of deterioration of original water quality | - / + | - / + | - | + | - / + |
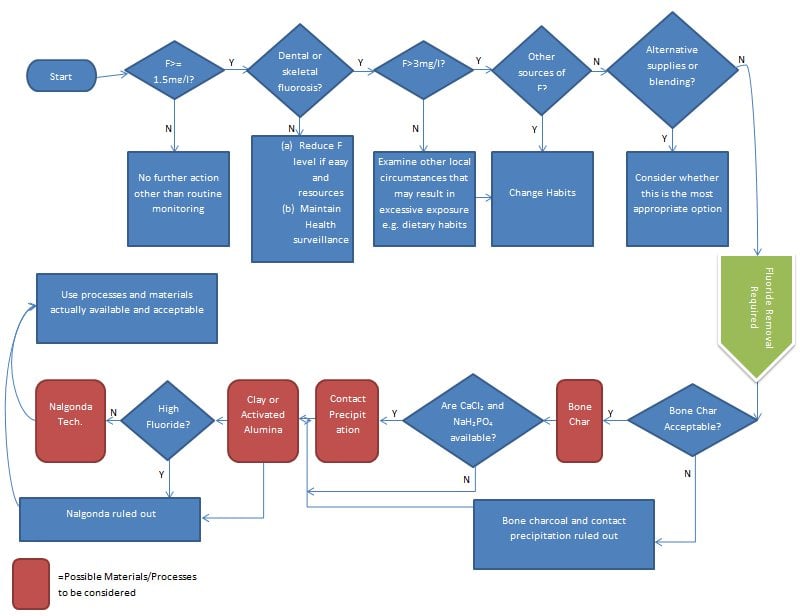
Additionally, the World Health Organization created the following flowchart to aid in the decision making process:[1]
Sources[edit | edit source]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 World Health Organization
- ↑ 2.0 2.1 Dahi, E. (1997). DEVELOPMENT OF THE CONTACT PRECIPITATION METHOD FOR APPROPRIATE DEFLUORIDATION OF WATER. 2nd International Workshop on Fluorosis Prevention and Defluoridation of Water, Nazreth, Ethiopia, The International Society for Fluoride Research.
- ↑ 3.0 3.1 K Bjorvatn*, A. B. a. R. T.-H. (1997). DEFLUORIDATION OF DRINKING WATER BY THE USE OF CLAY/SOIL. 2nd International Workshop on Fluorosis Prevention and Defluoridation of Water, Nazreth, Ethiopia, The International Society for Fluoride Research.
- ↑ 4.0 4.1 A Kvalheim*, K. B., A Bårdsen* and R Tekle-Haimanot (1997). SIGNIFICANCE OF ELEVATION ON FLUORIDE BINDING CAPACITY OF ETHIOPIAN SOILS. 2nd International Workshop on Fluorosis Prevention and Defluoridation of Water, Nazreth, Ethiopia, The International Society for Fluoride Research.
- ↑ Bjorvatn, A. B. a. K. (1997). FLUORIDE SORPTION ISOTHERM ON FIRED CLAY. The 1st International Workshop on Fluorosis Prevention and Defluoridation of Water, Ngurdoto, Tanzania, The International Society for Fluoride Research.
- ↑ E Dahi*, J. J. S., and J M Nielsen (1995). KINETICS OF DEFLUORIDATION OF WATER BY CALCINED MAGNESIA AND CLAY. The 1st International Workshop on Fluorosis Prevention and Defluoridation of Water, Ngurdoto, Tanzania, The International Society for Fluoride Research.
- ↑ 7.0 7.1 7.2 J J Singano*, D. A. M., F W Mtalo**, and E Dahi (1997). KINETICS OF SORPTION OF FLUORIDE ON CALCINED MAGNESITE IN BATCH. 2nd International Workshop on Fluorosis Prevention and Defluoridation of Water, Nazreth, Ethiopia, The International Society for Fluoride Research.
- ↑ JJ Singano*, D. M. E. D. a. F. M. (1995). EFFECT OF pH ON DEFLUORIDATION OF WATER BY MAGNESITE. The 1st International Workshop on Fluorosis Prevention and Defluoridation of Water, Ngurdoto, Tanzania, The International Society for Fluoride Research.
- ↑ Testing of a Defluoridating Filter Using MgO-CaO-CaCl2 for Use in Rural Rajasthan, MARGANDAN, K; AGRAWAL, R; SINGH, K; ACHARYA, R; SHARMA, S; QANUNGO, Kushal,Southern Brazilian Journal of Chemistry, Vol. 21, No. 21 p79-95 [1]
- ↑ C Venkobachar*, L. I. a. A. K. M. (1997). HOUSEHOLD DEFLUORIDATION OF DRINKING WATER USING ACTIVATED ALUMINA. 2nd International Workshop on Fluorosis Prevention and Defluoridation of Water, Nazreth, Ethiopia, The International Society for Fluoride Research.
- ↑ 11.0 11.1 11.2 11.3 E Dahi*, H. B. a. L. O. (1995). SORPTION ISOTHERMS OF FLUORIDE ON FLOCCULATED ALUMINA. The 1st International Workshop on Fluorosis Prevention and Defluoridation of Water, Ngurdoto, Tanzania, The International Society for Fluoride Research.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 Amir Haider, S. N., Qaisar Mahmood, et al. (2010). "Strategies for Low-Cost Water Defluoridation of Drinking Water-A Review of Progress." J.Chem.Soc.Pak. 32(4): 550-555.
- ↑ http://www.bibliotecapleyades.net/salud/salud_fluor23.htm
- ↑ N Lakshmaiah*, P. K. P., and P M Mohan (1997). BIODEFLUORIDATION OF FLUORIDE CONTAINING WATER BY A FUNGAL BIOSORBENT. 2nd International Workshop on Fluorosis Prevention and Defluoridation of Water, Nazreth, Ethiopia, The International Society for Fluoride Research.
- ↑ Anjaneyulu, L., Kumar, E. A., Sankannavar, R., Rao, K. K (2012) Defluoridation of Drinking Water and Rainwater Harvesting Using a Solar Still, Ind. Eng. Chem. Res. 51, 8040-8048.
- ↑ Anjaneyulu, L., Kumar, E. A., Sankannavar, R., Rao, K. K (2012) Defluoridation of Drinking Water and Rainwater Harvesting Using a Solar Still, Ind. Eng. Chem. Res. 51, 8040-8048.
- ↑ 17.0 17.1 17.2 Bapurao, S. (1997). FLUORIDE AND SILICON CONTENT IN DRINKING WATER. 2nd International Workshop on Fluorosis Prevention and Defluoridation of Water, Nazreth, Ethiopia, The International Society for Fluoride Research.
- ↑ L Mabelya*, M. v. t. H., WH van Palenstein Helderman* and KG K nig (The 1st International Workshop on Fluorosis Prevention and Defluoridation of Water). SUITABILITY OF THE TF-DENTAL FLUOROSIS INDEX FOR DETECTION OF FLUORIDE SOURCES, The International Society for Fluoride Research.