The biodiesel refinery at CCAT consists of five stages. The University of Idaho Department of Agriculture in 1991 defined making biodiesel, or transesterification, as the "process of using an alcohol (e.g., methanol or ethanol) in the presence of a catalyst, such as sodium hydroxide or potassium hydroxide, to chemically break the molecule of the raw renewable oil into methyl or ethyl esters of the renewable oil with glycerol as a byproduct (Making Biodiesel)." The process of making biodiesel will be described in the next three paragraphs.
Stage 1: Initial Filtering[edit | edit source]
Used vegetable oil from two different restaurants
Simple but effective screens
Once the used vegetable oil has been obtained it is filtered through a wire screen to remove all of the food particles. At CCAT we make 30 gallon batches in donated oil barrels (Thanks Spectrum!), and the filtered oil is then poured into a fifty gallon barrel. We begin heating the oil in the barrel to a temperature between 100-120 degrees F using a propane burner.
Stage 2: Making the catalyst[edit | edit source]
While the oil is heating we perform a titration using a 10 ml sample of the homogenous solution to determine the amount of lye (the catalyst) needed. Methanol in a standard 1/5 proportion to the amount of oil is combined with the determined amount of lye (sodium hydroxide) in a micro reactor to make sodium methoxide.
Here is the recipe and materials list for determining the amount of lye needed. This was taken directly from the folks at http://www.namecraft.com/dancing-rabbit/biodiesel:
You will need[edit | edit source]
- Used or fresh vegetable oil (strained with a coffee filter or cloth)
- Red Devil Lye Methanol (dry gas methanol-- we found it at automotive racing stores)
- Isopropyl Alcohol (for tests-- use 99% IPA)
- Eyedropper- or other type of 1 milliliter dropper
- pH paper-- available at drug stores-- to test for acidity
- An old blender plastic or glass measuring cups or beakers, with metric measures-- and/or a gram scale
- Plastic, glass or stainless stirrers and spoons
- Rubber gloves
- Safety glasses
- Plastic apron
Titration Process to determine how much Lye to use[edit | edit source]
Free fatty acids will increase with the amount of time vegetable oil has been heated-- oil which has been used for cooking will require more of the reactive agents-- lye and methanol-- than fresh oil. The presence of too many free fatty acids will retard or stop the reaction which produces biodiesel, so it is necessary to detect the exact amount of LYE (Sodium Hydroxide-- or NaOH) needed to neutralize the acids. Adding too much or too little NaOH will just make excessive amounts of By-product (soap).
- Dissolve 1 gram NaOH (Sodium Hydroxide- Red Devil Lye) in 1000 ml. of water. This is your NaOH solution.
- Dissolve 1ml. of Wasted Vegetable Oil (WVO) in 10 ml. Isopropyl Alcohol(IPA). This is your WVO solution.
- With an eyedropper, drop the diluted NaOH into WVO mixture a milliliter at a time. Count the drops. After each ml drop check the pH level of the WVO solution with standard pH paper-- you will see an eventual rise in the pH level. Continue to add the NaOH solution into the WVO solution, a drop at a time, until it reaches a pH of 8-9. To determine your proportions, figure: The number of drops of NaOH needed for the WVO solution to reach a pH of 8-9 1 ml. plus
- 5 g. NaOH to catalyze the oil.
An example formula used with one particular batch of WVO-wasted vegetable oil. 1 ml of oil was titrated with a 1g NaOH/1000 ml H2O solution. It required 6.0 ml to raise the pH level to 8 =.006g so 6.0g/1000ml to neutralize the free fatty acids plus 3.5 g NaOH as catalyst = 9.5 g per 1000ml oil or 9.5g/915g oil=103% by wt
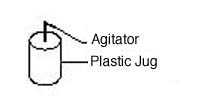
The determined amount of lye is added to the methanol in a separate plastic jug with a hole in the lid to allow the agitator to be powered by an attached drill. The drill attachment allows the sodium methoxide to be stirred without exposure to this extremely toxic chemical. If methanol is not available ethanol can be used instead, but more is required.
!!! Remember, the sodium methoxide you have just made is toxic and can do damage to your nervous system if it touches your skin. Take every precautionary step possible to contain and isolate the sodium methoxide and to prevent your unprotected skin from coming in contact with it !!!
The metal agitator fits into an electric drill head
The agitator CCAT uses to stir the methanol and lye mixture.
The agitator stirs the sodium methoxide in an isolated container for safety.
Stage 3: Mixing it all up[edit | edit source]
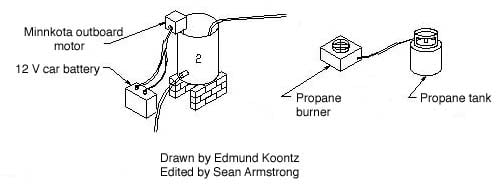
The sodium methoxide solution is then added to the heated oil. The propane burner is still required continue heating the oil for the next hour as the solution is stirred. At CCAT we use a small electric outboard motor to stir the solution. We found this to be the cheapest effective mixer for making 30 gallon batches of biodiesel.
The burner is place underneath the 50 gallon drum while the mixture is stirred for an hour
Stage 4: Settling down[edit | edit source]
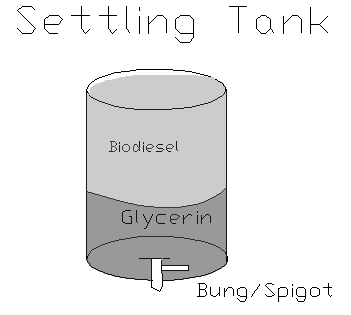
The solution in the 50 gallon barrel can then be drained or pumped into our settling tanks. The use of multiple settling tanks allows other batches of biodiesel to be made during the settling process or for storage. The settling process can take up to 24 hours and should result in distinct layering with the biodiesel (methyl esters) on top and the glycerin on the bottom. If distinct layering does not occur something went wrong and the biodiesel should not be used. Even if it does form layers, a specific gravity test with a hydrometer will tell you if it's a good batch. A specific gravity reading between.85 and.90 is successful, and anything outside of that window is a bad batch and should not be used in your car. Getting a hydrometer (it looks like a large thermometer and floats) requires a trip to your local chemistry supply shop or some good investigative work on the Web. Most hydrometers don't measure as low as.85.
Our two 33 gallon settling tanks
A diagram of how the solution settles out over the next 8 or more hours. It's not quite to scale--Biodiesel is about 80% and glycerin is about 20% of the finished volume
The biodiesel can now either be pumped off the top or it can be drained from the bottom. Pumping from the top was time intensive and difficult, so we took our barrels down to the local muffler shop and had bungs welded to the bottom so we could drain from below. If you drain the biodiesel from below the glycerin must first be drained out. It is easy to see the difference in viscosity when the glycerin is all poured out and the biodiesel begins to flow. Suddenly the liquid will be thin and running fast instead of the syrupy consistency of glycerin. This glycerin is biodegradable and nontoxic, so we compost it.
The gooey glycerin
Stage 5. Storage and final filtering[edit | edit source]
Once we have drained off the glycerin we pour the biodiesel into our storage tanks. Each of the tanks has a hand pump with an 10 micron in-line filter to remove any small bits of glycerin that didn't get removed. Since the not all of the materials may have reacted when you made the biodiesel (it's usually around 98% reacted), the filter may also remove oil or methanol.
The storage tanks with hand pumps and in-line filters
Use[edit | edit source]
There are some precautions you must take if using biodiesel in a vehicle. Biodiesel has the properties of a solvent, and it is recommended that if you have rubber hoses in the engine you should use a mixture of 40% biodiesel to 60% petrodiesel, or less. Replacing the hoses can be a hassle, but it may be easier in the long run if you plan to use biodiesel exclusively. It is perfectly all right to use biodiesel and standard petroleum diesel together in any proportion.
The solvent properties of biodiesel also have the unintended effect of cleaning out your gas tank! If you have an old engine with gunk floating around in the bottom of your gas tank you have two choices: either drain your tank completely before using biodiesel the first time (a good idea anyway just for the health of your engine) or expect to replace your fuel filter when the biodiesel cleans out your tank for you. Either way, once you deal with the junk in your tank you won't have to do it again as long as you continue using biodiesel.
At CCAT we are running a Mercedes station wagon, Volkswagen pickup truck, and a diesel generator on biodiesel. Aside from the issues listed above, we have had no problems and many benefits. The exhaust smells much better than petrodiesel (French fries!) and since it is made out of a soybeans, it is a makes no "net" addition to the amount of CO2 in our atmosphere. We "breathe easier" knowing we're not contributing to global warming. Below is a table showing the reduced emissions produced by even using biodiesel in a 20%-80% mixture.
| Total | Soluble | |||||
|---|---|---|---|---|---|---|
| Fuel | Particulate | (%) | CO2 | CO | NOx | THC |
| #2 EPA Diesel | 0.833 | 87.5 | 760 | 3.59 | 9.96 | 2.01 |
| 20%/80% Blend | 0.814 | 89.7 | 773 | 2.73 | 10.18 | 1.48 |
| %Change | ||||||
| 2.5 | 1.7 | |||||
| 2.2- |
Table 1: Emissions (DDC 6V-71N Emission Testing on Diesel and Biodiesel Blend)
The results of this study show that particulate matter and other emissions are reduced when a 20%/80% blend is used. However the amount of NOx and CO2 increased when this blend is used. But it's not a "net" increase of CO2.
Conclusion[edit | edit source]
Much of this system is based on the book From the Fryer to the Fuel Tank and should be referred to for specific information regarding the transesterification of used vegetable oil and for related issues such as cold weather use. A list of biodiesel related web sites are available online at veggievan.org. The basic materials needed to start a biodiesel refinery are readily available and are listed below.
Basic Materials for CCAT's system[edit | edit source]
- vegetable oil
- methanol
- lye
- 3 50 gallon drums (1 for reaction, 2 for storage)
- 2 30 gallon drums for settling process
- a pH kit (listed above in Stage 2)
- a stirring device for the sodium methoxide container
- a container for mixing sodium methoxide
- 1 outboard motor
- power source (12V battery)
- filters (window screen, cheese cloth, etc.)
- heat source (propane burner with tank)
- hydrometer
- hoses
- valves
- hand pump
- in-line 10 micron filter
Want to find out more?[edit | edit source]
Here are some sites where you can find more information about biodiesel and also more information on how to make your own biodiesel at home! These sites also have great links to even more biodiesel sites.
- http://www.nbb.org/fuelfactsheet.htm
- http://web.archive.org/web/20090401041619/http://www.veggievan.org:80/biodiesel/index.php
- http://www.biodiesel.org/reports/GEN-004.html
- http://www.make-biodiesel.org/index.php
Bibliography[edit | edit source]
- Biodiesel Production Technology Overview
http://www.biodiesel.org/reports/GEN-004.html
(February 17, 2000)
- Biodiesel
http://web.archive.org/web/20011117031840/http://www.baylor.edu:80/~rafdc/Biodiesel.html
(February 15, 2000)
- Frequently Asked Questions
(February 17, 2000)
- Tickell, Joshua and Kaia. From the Fryer to the Fuel Tank: The Complete Guide to Using Vegetable Oil as an Alternative Fuel 2nd ed. Green Teach Publishing
Transesterification: turning used vegetable oil into clean burning biodiesel fuel
http://web.archive.org/web/20090401041619/http://www.veggievan.org:80/biodiesel/index.php
(February 15, 2000)
- Why Biodiesel?
http://web.archive.org/web/20090401041619/http://www.veggievan.org:80/biodiesel/index.php
(February 15, 2000)
- Burning Biodiesel Fuel. Online. Internet.
(February 17, 2000)
Sendog6913 17:30, 2 March 2008 (PST) Cal Poly Humboldt - CCAT
Credits[edit | edit source]
By Sean Armstrong, Edmund Koontz and Celeste Peltier With special thanks to Andy Cooper and Panama Bartholomy (Spring 2000)