Hydrogen can be used as fuel in both internal combustion engines aswell as hydrogen fuel cells.
Hydrogen overview
Hydrogen is an atom. It contains one proton and one electron. On the Periodic Table Of The Elements, Hydrogen occupies the top spot - it has the Atomic Number of 1 and is the Atomic Element Number One-.
Deuterium is an Atom of Hydrogen that contains one neutron combined with the [single] proton in a Space that is "The First Atomic Nucleus". Tritium is an Atom of Hydrogen that contains two neutrons along with the proton in the Nucleus.
The H+ ion - also known as a proton - is exactly and only what makes Acids corrosive. Examples are [each has its own unique Strength] HBr, HCl, HF, HI, HCO3, H2SO4, all give off free protons when the Acid Substance is dissolved in Water (the aqueous solution). The Acid Subtance's strength depends upon its ability to give off free protons or H+.
The Hydronium Ion (H30+) is [next]:
Hydrogen production
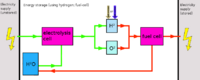
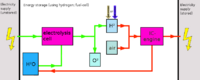
Due to the very difficult storability aswell as difficult production, hydrogen is best either converted to methane using the Sabatier process, or rather than using hydrogen, oxyhydrogen can be produced (as production is easier) and used rapidly (oxyhydrogen suffers from the same problems regarding storability).