Problem being addressed[edit | edit source]
Ultrasound imaging has proven crucial in antenatal and prenatal screenings however, women in low-resource settings often do not have access to such services due to financial strains and the fact that conventional ultrasound systems cannot be easily transported. As a result, preventable complications often go undetected. Thus, there is a need for cost-effective, portable, and efficient antenatal/prenatal screening services in developing nations.
Detailed description of the solution[edit | edit source]
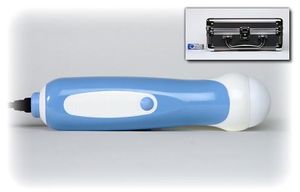
InNova Sound is an inexpensive portable ultrasound imaging system that is contained within a handheld probe that plugs into USB 2.0 ports of computers running Windows XP, Vista, or MAC operating systems. The device makes ultrasound available in nontraditional settings, bringing these services to those who need it the most.
Designed by[edit | edit source]
- Designed by: This product was designed by Direct Medical Systems, LLC.
- Manufacturing: This product is manufactured in the United States.
- Distribution: Applied Solution Group, Inc. Distribution centers are located around the world in the USA, Europe, Taiwan, and Australia. Link available here.
References[edit | edit source]
Other internally generated reports[edit | edit source]
Human Medicine Products. (n.d.). ASG Applied Solution Group, Inc. Retrieved January 1, 2013. Link available here.
Externally generated reports[edit | edit source]
InNovaSound portable ultrasound. (2012, August 23). Maternova: Tools and ideas that save mothers & newborns. Retrieved January 1, 2013. Link available here.
Personal Health: Direct Medical System's Plug and Play Ultrasound Probe System--Ultrasound on Your Laptop. (n.d.). Popsci's Best of What's New 2006. Retrieved January 1, 2013. Link available here.
Approval by regulatory bodies or standards boards[edit | edit source]
This device has been cleared by the FDA, 510k number: K070907 All manufacturing facilities are ISO and FDA certified.