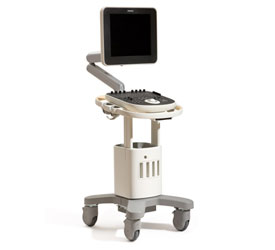
Problem being addressed[edit | edit source]
Maternal deaths in low-resource settings can oftentimes be prevented if women had access to antenatal care during pregnancy. Ultrasound imaging is especially useful to make accurate diagnosis for first trimester conditions such as ectopic pregnancy or gestational trophoblastic diseases. Many health centers in low-resource settings do not provide imaging equipment to provide screenings leading up to the birth, which ultimately leads to an increase in infant mortality.
Detailed description of the solution[edit | edit source]
ClearVue is a group of ultrasound equipment that provide cost effective yet high quality imaging that will allow clinicians to examine pregnant mothers during the term of their pregnancy. The device has a lightweight, modular, and energy efficient design, with low energy needs and easy serviceability.
Designed by[edit | edit source]
- Designed by: Philips Innovation Campus of Royal Philips Electronics
- Manufacturer (if different):
- Manufacturer location:
When and where it was tested/implemented[edit | edit source]
Field testing occurred in Ghana and India in 2012.
Funding Source[edit | edit source]
Royal Philips Electronics
References[edit | edit source]
Peer-reviewed publication[edit | edit source]
Other internally generated reports[edit | edit source]
Philips commits to strengthen healthcare infrastructure in Ghana; to open West African regional office in Accra. (2012, June 14). Retrieved from http://www.philipsafricaroadshow.com/philips-commits-to-strengthen-healthcare-infrastructure-in-ghana-to-open-west-african-regional-office-in-accra/
Externally generated reports[edit | edit source]
Singh, K. (2012, May 12). Philips launches ClearVue; to launch series of locally developed products electronics]. The Economic Times (Online). Retrieved from http://search.proquest.com.proxy.lib.umich.edu/docview/1012385230?accountid=14667