Since 1910 chlorine has been used in the disinfection process of wastewater because of its success in eliminating high rates of water-borne illnesses.[1]

Chlorine (Cl2) reacts with water (H2O):[2]
Cl2 + H2O → HOCl + HCl
The resulting hypochlorous acid (HOCl) disassociates into hydrogen (H+) and hypochlorite (OCl-) ions:
HOCl ---> H+ + OCl¯
Hypochlorous acid (HOCl) is a weak acid that penetrates the cell membrane of wastewater pathogens, destroying the enzymes within the cytoplasm. Wastewater pathogens killed through chlorination include bacteria, viruses, protozoa, and amoebic cysts.[3]
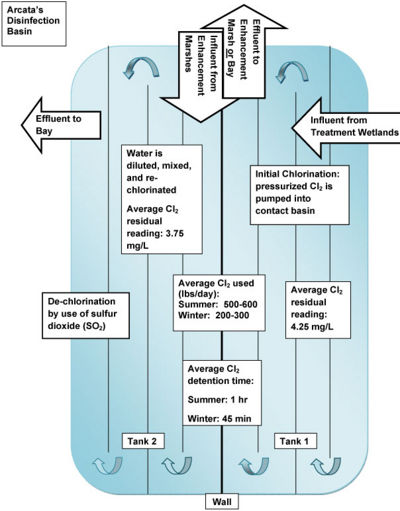
Chlorine’s effectiveness as a disinfectant depends upon:[3]
- chlorine detention time and concentration
- wastewater pH and temperature
- total suspended solids (turbidity)
- other reactive species in the water (hydrogen sulfide, organics)
- pathogen (cryptosporidium, for example, is highly resistant to chlorine)
- ↑ Water Quality and Health Council. Wastewater Chlorination: An Enduring Public Health Practice. Accessed online 5/02/08. http://www.waterandhealth.org/wastewater/chlorination.php3
- ↑ Chlorination. Accessed online March 27th, 2008. http://en.wikipedia.org/wiki/Chlorination
- ↑ 3.0 3.1 Davis, Mackenzie, and Susan Masten. Principles of Environmental Engineering and Science. McGraw Hill: New York, NY 2004.