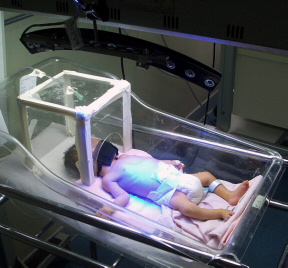
Problem being addressed
Neonatal jaundice, also known as hyperbilirubinemia, is a shockingly common condition that occurs when infants' livers are not fully developed. Low-resource settings often do not have the necessary devices to treat infants with neonatal jaundice because traditional phototherapy devices are too expensive for the developing world. They are also impractical due to frequent power outages.
Detailed description of the solution[edit | edit source]
BluLine Phototherapy uses LED technology to treat neonatal jaundice. The device includes a long lasting light blub and can be powered by a car or motorcycle battery. The LED technology consumes much less power than fluorescent tubes, and the battery offers 24 hours of power supply. Approximately the size of a boomerang, BluLine Phototherapy is also very portable.
Designed by[edit | edit source]
- Designed by: Duke University; PhotoGensis Medical
- Manufacturer location: Durham, NC
When and where it was tested/implemented[edit | edit source]
Being field tested in Honduras, Tanzania, and Nicaragua
References[edit | edit source]
Other internally generated reports[edit | edit source]
Photogensis Medical. (2008). Retrieved November 20 2013 from here.
Externally generated reports[edit | edit source]
Maternova. (January 11 2013). BluLine Phototherapy. Retrieved November 20 2013 from here.