Problem being addressed[edit | edit source]
Postpartum hemorrhaging is one of the most severe causes of maternal mortality. PPH (Postpartum hemorrhaging) rates in the developed worlds have severely decreased, but they are still a great threat in developing countries. The World Health Organization suggests that there are over 100,000 deaths due to postpartum hemorrhaging every year.
Detailed description of the solution[edit | edit source]
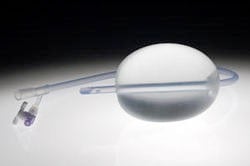
The Bakri SOS balloon is an obstetrical silicone balloon that has been approved by the US Food and Drug Administration. It was designed for one use only. The balloon is connected to a 24 French, 54 cm long silicone catheter. The balloon is inserted into the vagina in order to tamponade bleeding. There is a monitor that supervises excessive bleeding.
Designed by[edit | edit source]
- Designed by: Designer/Inventor: Younes N Bakri, MD. West Virginia University School of Medicine
- Manufacturer (if different): Cook Medical
- Manufacturer location:
References[edit | edit source]
Peer-reviewed publication[edit | edit source]
Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74(2):139-142. Bakri, Y. N., A. Amri, et al. (2001). "Tamponade-balloon for obstetrical bleeding." International Journal of Gynecology and Obstetrics 74(2): 139-142. Vitthala, S., I. Tsoumpou, et al. (2009). "Use of Bakri balloon in post-partum haemorrhage: A series of 15 cases." Australian and New Zealand Journal of Obstetrics and Gynaecology 49(2): 191-194.