Problem being addressed
- 8 million children under five died from respiratory complications in 2011, most in low-income countries. Continuous Positive Airway Pressure (CPAP) is a treatment for respiratory distress that could save the lives of many infants and preterm babies. However, the cost of conventional CPAP is prohibitive as it requires high, continuous flows of oxygen and air, resulting in high running costs that are unsustainable in a developing country. As a result CPAP is rarely available in developing countries, even in lifesaving situations.
Detailed description of the solution[edit | edit source]
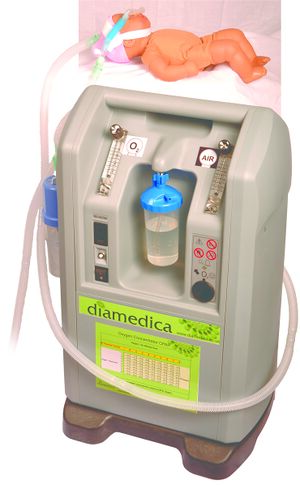
The Diamedica Baby CPAP machine has been designed for use in areas with limited resources. It is driven by an oxygen concentrator, thus eliminating the need for large quantites of expensive compressed gases. It provides controllable safe bubble CPAP with minimal running costs, for use with preterm babies, neonates and infants.
Designed by[edit | edit source]
- Designed by: Diamedica (UK) Ltd, Grange Hill Industrial Estate, Bratton Fleming, Devon EX31 4UH, United Kingdom http://www.diamedica.co.uk
- Manufacturer (if different):
- Manufacturer location: United Kingdom
When and where it was tested/implemented[edit | edit source]
Presently in use in ten countries: Afghanistan, Congo, Ethiopia, Kenya, Libya, Malawi, Papua New Guinea, Sierra Leone, Solomon Islands, Uganda