Traditional Field Crops (Peace Corps, 1981, 283 p.)
Pest and disease control
Weed control
How Weeds Lower Crop Yields
Numerous trials in the U.S. have shown maize yield losses ranging from 41-86 percent when weeds were not controlled. One trial in Kenya yielded only 370 kg/ha of maize with no weed control compared to 3000 kg/ha for clean, weeded plots. A CIAT trial with beans in Colombia showed a yield drop of 83 percent with no weeding.
Of course, all farmers weed their fields to some extent, but most of them could significantly increase their crop yields if they did a more thorough and timely job. A University of Illinois (U.S.) trial showed that just one pigweed every meter along the row reduced maize yields by 440 kg/ha. By the time weeds are only a few inches tall, they have already affected crop yields. Weeds lower crop yields in several ways:
- They compete with the crop for water, sunlight, and nutrients.
- They harbor insects, and some weeds are hosts for crop disease. (especially viruses).
- Heavy infestations can seriously interfere with machine harvesting.
- A few weeds like Striga (witchweed) are parasitic and cause yellowing, wilting, and loss of crop vigor.
Relative competitive ability of the reference crops: Slow starters like peanuts, millet, and sorghum compete poorly with weeds during the first few weeks of growth. Low growing crops like peanuts, bush beans, and bush cowpeas, however, are fairly effective at suppressing further weed growth once they are big enough to fully shade the inter-row spaces. However, tall-growing weeds that were not adequately controlled earlier can easily overtake these "short" crops if allowed to continue growing.
Some Important Facts on Weeds
Broadleaf versus Grassy Weeds
Broadleaf weeds have wide (broad or oval-shaped) leaves with veins that form a feather-like pattern. Grassy weeds are true grasses and have long, narrow leaves with veins that run up and down in a parallel pattern. A few weeds like nutsedge (nutgrass) belong to neither category, but are sedges, all of which have triangular stems. Some chemical herbicides are more effective on broadleaf weeds, while others give better control of grassy types.
How Weeds Reproduce and Spread: Annual versus Perennials
Annual weeds live only a year or so and reproduce by seed; they are the most common weeds in many fields. In the tropics, annuals may live more than a year if rainfall is sufficient. Most annuals produce tremendous amounts of seed, some of which may not germinate for years.
Rough Pigweed, Redroot (Amaranthus retroflexus)
An example of an annual broadleaf weed; reproduction is by seed.
Yellow Nutgrass (Cyperus esculentus)
An example of a sedge-type weed. The main stems of sedges are triangular in shape. This particular type reproduces by seed as well as producing underground "nuts," which sprout into new plants.
Bermuda Grass, Devilgrass (Cynodon dactylon)
An example of a perennial grassy weed; reproduction is by above-ground runners called stolons as well as by seeds.
When the soil is stirred with a hoe, harrow, or cultivator to kill weeds, one crop of them is destroyed, but more weed seeds are brought closer to the surface where they can sprout.
Annual weeds should be controlled before they produce seed. Even so, permanent eradication of annual weeds is not possible because most fields contain millions of weed seeds waiting to germinate, and the supply is continually replenished with more seeds brought in by wind, water, animals, animal manure, and contaminated crop seeds.
Perennial weeds live more than two years. Most produce seed, but many also propagate by means of creeping, above ground stems (stolons), and creeping underground stems (rhizomes). Hoeing or mechanical cultivation may actually aid in spreading them around the field.
Many herbicides will kill only the top growth, and there is usually enough food in the underground parts to continue propagation.
Identifying Weeds
Where weeds are being controlled by hoeing or mechanical cultivation, their specific identification is usually not important. Where chemical weed control is used, however, the farmer and extension worker should have a good idea of which specific weeds are present since herbicides do not give broad-spectrum control. (See bibliography for sources of further information on weed identification.)
Weed Control Methods
Burning
When land is cleared by burning, standing annual weeds are killed along with weed seeds very near the soil surface. However, burning will not kill weed seeds or reproductive underground parts of perennial weeds if they are deeper than 4-5 cm. Furthermore, as the brush is often placed in windrows or piles before burning, much of the soil may not be affected by the fire. Some perennial tropical grasses such as Guinea (Panicum maximum) and speargrass (Imperata cylindrica) are actually stimulated into dense regrowth by burning. On the other hand, weeds may be less of a problem under slash and burn farming, because the soil is usually not turned by plowing or cultivation which brings more weed seeds to the soil surface.
Mulching
Mulching the soil surface with a 5-10 cm layer of crop residues, dead weeds or grass can give very effective weed control and provide a number of other benefits:
- Erosion is greatly reduced on sloping soils.
- Soil water loss by evaporation and runoff is greatly reduced.
- In very hot areas, soil temperatures are reduced to a more beneficial level for crop growth.
- Organic matter is eventually added to the soil. In trials conducted by IITA in
Nigeria, mulching increased maize yields by 23-45 percent and greatly reduced the heavy labor requirement for hand weeding which accounts for 50-70 percent of the hours needed to grow maize in that area.
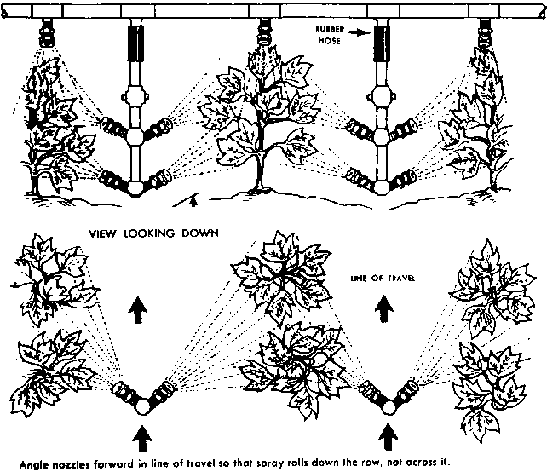
Shading (The Row Crop Principle)
Arranging crops in rows facilitates hand weeding, but also makes possible mechanical cultivation (weeding) with tractor or animal-drawn equipment. In addition, the rows permit the crop to exert better shade competition against the weeds.
Hoe and Machete Cultivation
Weeding with hand tools is an effective method if sufficient labor is available. It is common, however, for small farmers who rely on this method to fall behind in weeding and crop yields often suffer.
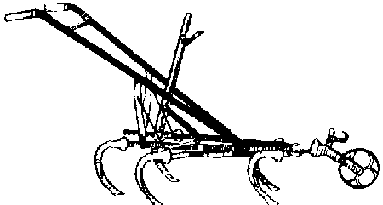
Animal and Tractor-drawn Cultivation
Disk harrows, field cultivators, and spike-tooth harrows can provide excellent pre-planting weed control. The spike-tooth harrow can also be used to control emerging weeds until the crop is about 7.5-10 cm tall. without serious damage.
Animal-and tractor-drawn row cultivators can be used from the time the crop is a few centimeters tall.. They are faster than weeding by hand, and a one-row animal-drawn model can easily cover 3-4 ha/day unless the rows are very narrow. They can also be adjusted to throw soil into the row itself to kill small weeds by burying them. If operated too deeply or too close to the row, however, serious root pruning (cutting off crop roots during cultivation between rows) may result.
Herbicides
Herbicides can greatly reduce labor requirements and permit a farmer to grow a larger acreage. They also avoid root pruning, soil compaction, and stand reduction which are caused by hand tools or mechanical equipment. In a number of cases, herbicides like atrazine have proven competitive with hand labor in maize production in developing countries. Improved methods for small farmer application of herbicides such as granular forms and ultra low volume sprayers are being developed by IITA. Herbicides do have some very definite disadvantages that must be considered when working with small farmers:
- They are less reliable than hand tool or mechanical weeding and most require careful and accurate application. This can be achieved by small farmers using backpack sprayers, but it requires some training.
- Weed control is seldom complete. Most herbicides are not broad-spectrum, and it is important to analyze the type of local weeds species present before choosing a product.
- Most soil applied herbicides require a certain amount of rain within a week after application in order to move the chemical into the zone of weed seed germination. Others need immediate incorporation into the soil with a disk harrow or rototiller.
- Improper application may damage the crop.
- Nearly all herbicides are unsuited for use in inter-cropping involving cereals and legumes due to the danger of crop injury. These products are crop-specific as well as weed-specific.
- Without proper training and care, farmers may subject themselves and the environment to serious risks through the misapplication or mishandling of these toxic chemicals.
Guidelines for Non-Chemical Weed Control in the Reference Crops
Pre-planting Weed Control
Successful weed control begins with planting the crop in a seedbed free of standing or emerging weeds. This means that when planting on tilled ground (as opposed to pure slash and burn agriculture), the field should undergo some form of cultivation (i.e. plowing, harrowing, hoeing, etc.) as close as possible to planting. This will give the young seedlings a "head start" on future weeds which is especially important under two conditions:
- Slow starters like sorghum, millet, and peanuts: They are very vulnerable to early season weed competition.
- Reliance on tractor or animal-drawn row cultivation: The only way these cultivators can control weeds in the crop row is by throwing in soil to bury them. This means waiting until the crop is tall enough (usually over 5 cm) so that it will not be buried too. The problem is that weeds already present or about to emerge in the row at planting may be able to grow tall enough to escape burial by the time cultivators can be used.
Frequent pre-plant harrowings do little to reduce the field's potential weed population and they can increase soil compaction and destroy good filth by speeding up the loss of organic matter.
How to Use a Spike-Tooth Harrow on Young or Emerging Seedlings
If large numbers of weeds emerge at the same time as the crop, a shallow working of the entire soil surface (including the rows themselves) with a spike-tooth (pegtooth) harrow may be the best solution if hand weeding labor is inadequate or too expensive. This method is best suited to crops planted at least 40-50 cm deep and can be used any time from two to three days after planting until the crop is 7.5-10 cm tall.
Peanuts and beans, with their brittle stems, are more likely to be injured than maize and sorghum, unless certain precautions are taken (see below). Millet is usually planted too shallowly to tolerate this method well.
Guidelines for using the spiketooth harrow for this type of weeding:
- The weeds should be either just emerging from the soil or still very small.
- If the soil is very wet and the weather is cloudy, the weeds may be transplanted instead of killed.
- The harrow should be run only deep enough to uproot the tiny weed seedlings.
- Beans and peanuts are more easily injured when they first emerge and still have the crook (bend) in the stem.
- Less injury is likely if the harrow is used in the afternoon when the plants are less turgid (hard) and brittle.
- Care must be taken to ensure that the draft animal or the tractor tires do not run over the row itself.
Using the spike-tooth harrow in this manner once or twice can often eliminate future, more laborious weeding. Use of this harrow prior to plant emergence is also useful for breaking up any soil crusting that might hinder emergence. (For more information on the spike-tooth harrow, see the PC/ICE Animal Traction manual.)
Guidelines for Animal and Tractor-Drawn Row Cultivators
Animal-drawn cultivators are widely manufactured in one-row models and cost about - in U.S. currency. They are well worth the investment since they permit more timely and rapid weeding than is possible with hand tools. A one-row cultivator can easily weed 2-3 hectares per day of wide row crops such as maize, millet, and sorghum. Animal-drawn models are available either as single-purpose units or as multi-purpose toolbar frames with attachments for plowing, ridging, and cultivating.
Tractor-drawn cultivators usually consist of a toolbar to which cultivating shanks are attached. Two-row, four-row, six-row, and eight-row arrangements are most common. It is important to remember that such multi-row arrangements require uniform spacing of the planting rows to avoid crop injury.
Cultivator Shovels and Sweeps: Both animal- and tractor-drawn cultivators use sweeps and/or shovels attached to the cultivator shanks to do the actual weeding. Some important considerations:
- Shovels require deeper soil penetration for good weed control and throw more soil than most sweeps. This means that in the case of tractor usage, shovels cannot be operated as close to the crop rows as fast as most sweeps.
- Sweeps are available in widths up to about 50 am. However the farmer is usually better off using two or more sweeps of smaller widths or a combination of sweeps and shovels to cover one inter-row space. This permits more effective weeding and more accurate adjustment than is usually possible with just one wide sweep. Wide sweeps are also more prone to breakage.
An animal-drawn cultivator which can be adjusted for width by moving the upright level.
Some General Guidelines for Weeding with Row Cultivators
1. A sure sign of root pruning is the accumulation of crop roots on the cultivator shanks. To avoid serious root pruning, shovels and sweeps should be operated as shallowly and as far from the crop row as practical. The ideal depth and distance will vary with crop size and row width. For example, when maize is 20 cm tall, it can be cultivated up to 10-15 cm from the stalks. However, once the crop is 75 cm tall, such deep cultivation would prune off much of the root systems. Maximum depth should be about 5.0-7.5 cm at this stage. Sweeps can be run shallower and closer to the row than shovels and do a good job of weeding without root damage.
2. Sweeps should be set to operate almost flat with the tips angled just slightly downward. When the point rests on a floor or the ground, the outside tips of the wings should rest about 30-60 cm off the surface.
3. Weeds should be killed early to avoid yield losses and to permit more effective control, especially of weeds right in the row.
4. The nitrogen side dressing is best applied right before a cultivation, then the fertilizer can be worked into the ground a bit to prevent losses through water runoff or through conversion into ammonia gas (a problem with urea).
5. Cultivation is most effective when the soil surface is dry; wet soil keeps partially uprooted weeds alive.
6. The cultivator should be adjusted so that it throws sufficient soil into the crop row to bury small weeds without smothering the crop. DO NOT THROW SOIL INTO PEANUT ROWS (see page 248)
7. Unnecessary cultivation can harm the crop. The main purpose of cultivation is to control weeds, although it is sometimes used to break up a hard soil crust that is interfering with water absorption. Excessive cultivation damages plants and roots, wastes time and labor, and increases soil compaction and loss of humus.
Different types of cultivator shovel; note that some have reversible points.
Different types of sweep. They come in many widths. The height of the sweep's crown determines how much soil it throws. The halfsweeps are used next to the crop row to help avoid damage.
Guidelines for Cultivating Reference Crops
MAIZE AND SORGHUM: In many regions, these two crops are commonly "hilled up" during successive cultivations to provide better drainage and to help prevent lodging.
BEANS: Throwing soil into the plant row not only controls small weeds and provides better drainage (good for root rot control), but also helps promote the growth of secondary roots. This is especially beneficial in cases where the primary root system has been damaged by root rot. Do not cultivate beans while the leaves are wet since this increases the spread of foliar diseases like bacterial blight and anthracnose.
PEANUTS: Soil should not be thrown into the crop row, especially when the peanut plants are young. This practice injures the stems and buries some of the young branches which greatly increases the plants' susceptibility to Southern stem rot (Sclerotium rolfsii) and also interferes with normal branch development. There is no need to throw soil into the row if early season weed control is adequate.
"Flat" cultivation will avoid throwing soil into the row. The secret of flat cultivation is good early-season weed control to prevent weeds in the row from overtaking the crop. Most farmers in the U.S. use herbicides to provide initial control for the first six to eight weeks. If using tractor cultivators, farmers should use "high speed" sweeps which have a low crown and do not throw as much soil. Wide sweeps enable the cultivator shanks to be kept well away from the row since they, too, throw a lot of soil.
Cultivation should cease once the pegs begin to elongate, around eight weeks after plant emergence. Cultivation at this stage can damage the pegs and help spread rosette virus, a serious problem in Africa. By this stage, the plants should be big enough to provide good competition with any emerging weeds.
A Special Note on Striga
Striga (witchweed) is a parasitic annual weed which invades the roots of grass family plants (sorghum, maize, millet) and can cause serious losses. There are several species found in Africa, India, Southeast Asia, Australia, and the Southeastern U.S. In West Africa, improved varieties of sorghum are sometimes heavily attacked. Improved maize varieties are somewhat less susceptible but native varieties have better resistance. Gero type millet usually escapes injury since it is harvested during the wet season when striga seeds are dormant. Maiwa millets, which mature later, are more prone to attack. Striga seeds are stimulated to germinate by moisture and plant juices (root excretions) from the roots or grass family host plants and emerge above ground in about one to two months. Flowering occurs three to four weeks later, and the seeds mature in another 30 days. A single plant can produce half a million seeds which are easily spread by wind, water, and tools. Crops are often injured before the weed emerges, and severe attacks cause stunting, yellowing, and wilting.
Striga Control Recommendations
- Hand weeding provides partial control; some herbicides give good control, and one foliar product has been developed that can be applied with an inexpensive water pistol.
- High fertility helps plants resist attacks, and plant breeders are working on varietal resistance.
- An effort should be made to prevent movement of striga seed from infested to non-infected fields.
- All crops should be kept free of grassy weeds which are hosts for striga.
- "Trap" crops of cereals or grasses can be planted to stimulate striga germination and then plowed under before the weeds have produced seeds.
Guidelines for The Use of Herbicides in the Reference Crops
In some parts of the developing world, there is a critical labor shortage at weeding time. Herbicides can be economically feasible for small farmers under these conditions. In Central America, herbicide use by small farmers has become common in many districts. Chemical weed control is a sophisticated management practice, however, and most farmers using herbicides need more instruction in proper application procedures.
How Herbicides Kill Weeds
Some herbicides like glyphosate will kill weeds only if sprayed on their leaves. Others like simazine will not control emerged weeds, but must be applied to the soil itself where weeds are killed as they germinate by absorbing the chemical through their roots. Some herbicides like atrazine are effective either way.
Choosing a Herbicide
The choice of a suitable herbicide depends on the type of weeds present and the crop's tolerance to the chemical. Weed Selectivity: Some herbicides control grassy weeds better, some are more effective on broadleaf types, and still others will control some of each. Nearly all herbicides are much more effective on annual weeds than perennial weeds. It is important to remember that individual herbicides seldom provide a full range of weed control and that the specific weed species must be considered when choosing a product to control it.
Crop Tolerance: Each crop may tolerate certain herbicides, but at the same time, be severely injured or killed by others. For example, atrazine will kill most annual grassy and broadleaf weeds on maize, sorghum, and millet without injury to the crop. The herbicide 2, 4D can also be sprayed directly on maize, sorghum, millet, and other grass family crops to control broadleaf weeds without injury to these crops (unless applied too heavily or at the wrong stage of growth). On the other hand, glyphosate has no selectivity and will kill all foliage that it touches.
Some Important Herbicide Terminology
Contact herbicides kill only those plant parts the spray actually touches. There is little, if any, translocation (movement) to other parts of the plant. Contact herbicides can be either selective or non-selective.
Glysophate is a non-selective contact product that kills the green top growth of all weeds and crops. Propanil is a selective contact herbicide that controls many grassy and broadleaf weeds in rice without injury to the crop (it can be freely sprayed on the rice plants).
Systemic herbicides are absorbed through the leaves (less so through the roots) and then translocated throughout the plant. Systematics are especially useful for killing perennial weeds, although several applications may be needed. Many other herbicides like atrazine have a partial systemic action.
Timing and Method of Herbicide Applications
The herbicide label will state that the particular product can be applied in one or more of three ways:
- Pre-plant: Before the crop is planted. Most pre-plant herbicides require incorporation into the top 2.5-10 cm of soil with a disk harrow or rototiller.
- Pre-emergence: After the crop is planted, but before it or the weeds have emerged.
- Post-emergence: After the crop and the weeds have emerged, usually before the weeds are 2.5-5.0 cm tall.
Broadcast applications are applied over the entire field. Band applications are applied in a narrow strip (about 30-40 cm wide) centered over the crop row. These save the farmer money since less herbicide is used, but he or she will still have to cultivate the untreated inter-row area.
How Herbicide Dosages are Given
Herbicide recommendations are usually given in terms of lbs./acre or kg/ha of active ingredient* which refers to pure 100 percent chemical. However, each herbicide is usually available in several different formulations (i.e. wettable powders, liquids, granules) that vary in strength. It is up to the farmer or extension agent to figure out how much of a particular product is needed to satisfy the recommendation. This is much the same as figuring fertilizer requirements. For example 3.75 kg/ha of Gesaprim 80 percent wettable powder would be needed to supply 3 kg of active ingredient per hectare (80 percent x=3 kg; x=3.75 kg).
Herbicide Safety
Fortunately, most herbicides are relatively safe, but there are a few exceptions:
- Paraquat has an unusually high oral toxicity and even a small amount of diluted mixture can be fatal. Paraquat is inactivated by clay or activated charcoal which should be administered orally (mixed with water) if oral ingestion occurs.
- Dinitrophenols (DNBP, Dinoseb, Basanite) have high oral toxicity and can also be absorbed dermally (through the skin) .
- Suspected birth defects caused by 2, 4-D type herbicides have been linked with faulty manufacture which produces dioxins (rarely present under current production methods).
For these reasons, it is not recommended that these herbicides be used without first receiving instructions in handling from a knowledgeable professional.
The same general safety guidelines in section B. On insecticides apply to herbicides. Except for those mentioned above, nearly all herbicides are Class 4 in their relative toxicity (least dangerous).
Factors Affecting Herbicide Performance
- Choice of product: The product must be suited to the crop and the weed species present.
- Soil organic matter and clay content: The rates of most soil-applied herbicides are very dependent on soil clay and especially organic matter content. The higher these levels, the higher the rate of herbicide needed. Some soil-applied herbicides may cause crop damage on sandy soils.
- Rainfall: Most pre-emergence herbicides require moderate rainfall within a few days following application in order to move the chemical into the weed seed germination zone. Otherwise, a very shallow cultivation may be needed to work the chemical into the soil.
- Weed size: Post-emergence applications of many herbicides will not kill weeds much taller than 2.5 cm while others will effectively control larger weeds.
- Accuracy of application: Most herbicides need to be applied at fairly precise dosages. This requires calibrating the sprayer in order to determine how much water it will take to cover the field and how much herbicide should be added to each tankful. When spot spraying, the farmer can get by using a tablespoon per gallon or cc per liter dosage, but this is the exception. Application also needs to be uniform to avoid crop injury or patches of surviving weeds.
General Guidelines For Applying Herbicides
- READ AND UNDERSTAND THE LABEL!
- Do not spray on windy days. Spray drift or vapors may damage nearby susceptible crops.
- Avoid spraying when the temperature is above 32 High temperatures increase volatility (vaporization) and may also reduce herbicide effectiveness.
- When using wettable powder formulations, be sure to agitate the sprayer tank to keep the powder in suspension during application.
- Never use a herbicide on a crop for which it is not recommended.
- Do not burn herbicide containers. Fumes may be released which can injure susceptible crops.
Herbicide Carryover
Some herbicides take a long time to break down in the soil and may injure succeeding crops. It is likely that residues may cause problems with those crops for which the product is not recommended. Fortunately, residues are less of a problem in the tropics where higher temperatures favor a more rapid breakdown of the chemicals. Atrazine takes two to eight months for its residues to disappear, and most broadleaf crops may be injured if planted within this period. Simazine, diuron, and diphenamid may take even longer. Most others take a few weeks to a couple of months. The label should show carryover information.
Applying Herbicides with Backpack (Knapsack) Sprayers
A few herbicides do not require much dosage accuracy and can be easily applied with backpack sprayers. However, most herbicides require a level of precision, which is difficult to achieve with these sprayers unless extra care is taken.
In order to avoid applying too much herbicide, which wastes money and might injure the crop, or too little, which might make the spraying ineffective, the sprayer should be calibrated (see Appendix K).
Once the sprayer has been calibrated, the farmer must maintain the same constant spraying pressure and walking speed that was used in the calibration process.
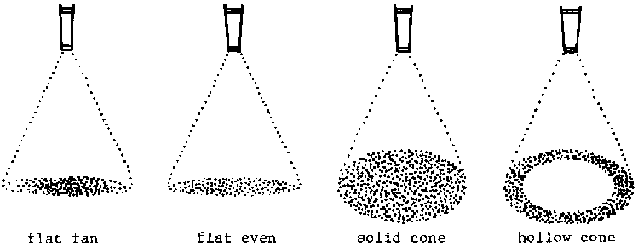
Nozzle selection is important. Fan nozzles (see page 225) should be used to make pre-emergence and postemergence applications over the soil and small weeds. Cone nozzles are best for spraying herbicides on larger weeds, since they provide more complete coverage than fan nozzles when used on foliage. They should not be used for broadcast applications of herbicides over the soil and small weeds since the circular spray patterns will not overlap properly. If two or more cone nozzles are mounted on a spray boom, overlapping spray patterns will distort each other. As for water volume, 250-300 1/ha is adequate as long as weeds are small or only the soil surface is being sprayed. Larger weeds require up to 500-600 1/ha when uniform coverage is needed. The sprayer should be shaken periodically to keep wettable powder formulations in solution.
Improvements in Hand Sprayers
- Low-volume hand-held sprayers: A very effective hand-carried sprayer that runs on flashlight batteries has been developed by IITA. It is known as a controlled droplet applicator sprayer and is specifically designed for applying herbicides. Its special nozzle produces extremely fine droplets which permit adequate coverage to be achieved with only 20 liters of water per hectare. The single nozzle covers a meter-wide swath which enables a hectare to be sprayed in about eight hours at a walking speed of 0.5 meters/ second. This is a big improvement over backpack sprayers in terms of water volume and time requirements. The controlled droplet applicator sprayer is very light and holds just 2.5 liters of spray solution. Calibration is also simplified, because the sprayer's output is constant and only walking speed need be considered. The sprayer is currently being manufactured by two companies:
- The "HERBIE" by Micron Sprayers Ltd., Bromyard, Herfordshire, ENGLAND HR7 4HU. This model uses eight flashlight batteries (good for up to five hectares of spraying).
- The "HANDY" by Ciba-Geigy AG, CH 4000, Basle 7, SWITZERLAND. Uses five flashlight batteries.
The price of the controlled droplet applicator is about half that of a backpack sprayer. However, it is not suitable for applying most insecticides and fungicides.
A spray boom for backpack sprayers: To reduce labor requirements for backpack spraying, a simple but effective spray boom can be constructed so that two to five nozzles can be used at once. If only two nozzles are used, special "T" extensions are commercially available for many sprayer models. Larger booms can be made by arranging nozzles along a length of narrow diameter pipe and connecting them with high-pressure plastic hose. If fan nozzles with an 80° angle of spray width are used and spaced 50 cm apart on the boom, uniform ground coverage can be achieved when the boom is carried about 50 cm off the ground. (This provides three to four fingers width of overlap between adjacent spray patterns. As shown in the illustration, these large booms are too unwieldy to be carried by the sprayer operator alone.
Applying Herbicides with Tractor Boom Sprayers
Tractor boom sprayers can cover up to six to eight rows at once and have nozzles spaced every 40-50 cm. They may be used on small farms as part of a cooperative venture. Here are some guidelines:
1. Low sprayer pressures (30-40 1bs./ sq. in.) are usually recommended for herbicides. Higher pressures decrease droplet size, distort the spray pattern, and cause drift.
2. For nozzle selection follow the guidelines listed under backpack sprayers. Brass, aluminum, and plastic nozzle tips are cheapest. However, they wear much faster than tips made of harder metals when wettable powders are used.
3. If output per nozzle is too low, switch to a larger nozzle size or drive slower. Increasing pressure is a poor way of increasing spray volume. Pressure must be increased fourfold in order to double the output.
4. When broadcasting herbicides over the soil or on very small weeds, the sprayer boom height should be adjusted to give three to four fingers width of overlap between adjacent spray patterns. Fan nozzles are available with different spray width angles such as 65°, 73°, and 80 . The wider the angle, the closer to the ground the boom can operate and still achieve the necessary overlap. This is a big advantage on windy days.
5. Nozzles of different sizes or spray angles should not be used on the same boom.
6. The manufacturer's tables for output and calibration are not reliable. Nozzle output can be markedly affected by wear, and pressure gauges and tractor speedometers vary in accuracy.
7. Wettable powder formulations need constant agitation to stay in suspension. Mechanical or hydraulic jet agitation is a must for tractor sprayers.
8. The tractor must be driven at a constant speed while spraying or output will be affected. A fluctuation of only 1-2 km/hr can increase or decrease the dosage being applied by as much as one third.
9. Tractor speed should be adjusted to suit ground conditions. Excessive bouncing of the spray boom will cause uneven coverage. The tractor should not be driven faster than 8 km/hr.
10. It is important to check constantly for blocked nozzles while spraying.
RECOMMENDED HERBICIDES FOR THE REFERENCE CROPS
The number of herbicides available for use on the reference crops and their individual application guidelines are too numerous to be adequately covered in this manual. It is best to rely on locally-derived recommendations based on field trials if possible. Several resources are listed in the bibliography that will provide reliable general guidelines for herbicide selection and dosages.
Insect control
Some Important Facts on Insects
Insects can often be identified by the type of damage they cause:
- Chewing and Boring Insects
Caterpillars are the larvae of moths. They damage plants by feeding on leaves and making holes in them or by boring into stalks, pods, and maize ears. The cutworm caterpillar is unusual in that it lives in the soil and emerges at night to cut off plant stems near ground level.
Bettles feed on plant leaves and chew holes in them. Some beetles of the weevil family bore into pods and seeds and deposit eggs inside. Certain beetles can also transmit bacterial and viral diseases.
Beetle larvae like white grubs, wireworms, and rootworms live in the soil and damage roots and the underground portion of the stem by chewing or boring.
- Sucking Insects
Aphids, leafhoppers, stinkbugs, harlequin bugs, whiteflies, and mites have piercing and sucking mouthparts and feed on plant sap from leaves, pods, and stems. They transmit a number of plant diseases, especially viruses. Sucking insects do not make holes in the leaves, but usually cause leaf yellowing, curling or crinkling.
Insect Life Cycles
A general understanding of insect life cycles is useful in identifying insect problems in the field. Beetles and moths go through a complete metamorphosis (change in form) consisting of four stages, while aphids, leafhoppers, whiteflies and other sucking insects go through only three stages.
(Adult stage)
MOTH � EGG �
(Does no damage.)
|
CATERPILLAR |
� |
PUPA |
|
(Usually feeds on leaves.) |
(Dormant stage; turns into a moth.) |
(Adult stage)
BEETLE � EGG
(Feeds on leaves,pods)
|
LARVA |
� |
PUPA |
|
(Grubs, wireworms, rootworms, etc. Feed on plant roots.) |
(Dormant stage turns into a beetle.) |
(Adult stage)
APHIDS, LEAFHOPPERS,STINKBUGS, WHITEFLIES, OTHER SUCKING INSECTS �
EGG � NYMPH
(Looks like a miniature adult; at this stage also sucks sap.)
How to Identify Insects and Their Damage
BE OBSERVANT! Troubleshooting takes practice, and a sharp eye is essential. When walking through a field, closely examine the plants for insects or their damage symptoms. Check both sides of the leaves since many insects prefer the undersides of leaves. A magnifying glass can be very helpful.
Identifying Insect Damage: Often it is possible to identify insects by the damage they cause.
- Holes in leaves: Caused by caterpillars, beetles, crickets, snails, and slugs. (Snails and slugs are not insects but do attack plant foliage.)
- Wilting: Usually caused by soil insects like white grubs and wireworms. If root feeding or tunneling of the underground portion of tile stem has been serious it could be due to stem borers. Remember that wilting can be caused by other factors, too: dry soil, very high temperatures, root rots, bacterial and fungal wilts, and nematodes.
To determine if insects are the cause of wilting, dig up the affected plants. Check the root system and underground portion of the stem for insect and disease damage, also look for soil insects. Slit the stem lengthwise with a pocket knife and check for borers or rotted tissue.
- Leaf curling, crinkling or yellowing: Caused by sucking insects, especially aphids, leafhoppers, and mites. Viruses and some nutrient deficiencies also produce these symptoms. Nematodes and poor drainage cause yellowing too.
Identifying Insects: Spend time with locally experienced exten ion workers in the field and have them point out the prevalent crop insect pests (and beneficial predator insects) in the work area. Seek out host country or regional insect guides such as extension bulletins. The publications listed in the bibliography are also very useful.
Major pests of the reference crops
This list is not complete but deals with the more prevalent reference crop pests. Full or partial (genus only) scientific names are given in parentheses. More specific control measures will be given at the end of the insect section. Stored grain Pests, some of which attack the crops before harvest, will be covered in Chapter 7.
Major Pests Of Maize
- Soil Insects
White grubs (Phyllophaga, others): Brown headed, plump, six-legged, white larvae up to 25 mm long. Many are larvae of May (June) beetles and attack roots of maize and other grass family crops, sometimes causing serious damage. Especially common where maize is planted on recently cleared pasture land. Occasionally attacks legumes. Larval stage lasts one to three years.
Rootworms (Diabrotica, others): Small, slender, whitish larvae with brown heads, measuring up to almost 20 mm. They attack the roots and sometimes bore into the underground portion of the stalk while adult beetles feed on the silks and attack other crops. They are most prevalent in Latin America. Affected plants often become "goosenecked" because of lodging caused by root damage. Ten or more larvae per plant or a brown discoloration of 50 percent of the root system indicates serious damage.
Wireworms (Elateridae): Shiny, brown, hard larvae up to 1.5 - 3.5 mm long with six legs. The larval stage of click beetles attack germination seeds and below ground plant parts. Larval stage lasts two to six years.
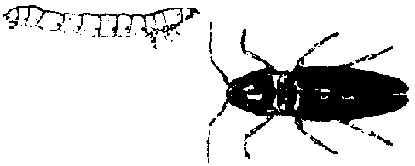
Cutworms (Agrotis, Feltia, Spodoptera): These are caterpillars ranging from bright green to black. Most are rather plump and curl up when disturbed. They attack young plants and cut off stems at or slightly above the soil surface, but some will feed on the leaves. Most remain below ground during the day and emerge at night to feed.
Lesser cornstalk borer (Elasmopalpus: Caterpillars, usually light green with faint stripes and distinct vertical bands of brown. They are most common in Latin America. Young larvae feed first on the leaves and then bore into the stalk about 2-5cm above ground. Each builds a tunnel made of soil particles and silk that runs from the soil to the stalk hole. May also attack the root system. Larval stage lasts about three weeks and pupation takes place in the soil in a silken cocoon.
Seed corn maggots (Hylemya): Yellowish gray fly larvae up to 6-7 mm long with a blunt rear end and a sharply-pointed head. They attack germinating seeds, sometimes eating out the entire kernel.
- Maize Foliage Insects and Borers
Fall armyworm (Spodoptera frugiperda: Larvae have a green and brown coloring with a prominent, white, inverted "Y" mark on the head and grow to about 40 mm. One of the most serious and prevalent maize insects in the lowland tropics. The caterpillars are larvae of night-flying moths that lay eggs in clusters of 100 or more on the leaves. Eggs are covered by a coating of body hairs and scales and hatch in two to six days in warm weather. The larvae are cannibalistic and attack each other until only a few are left. They then move to the leaf whorl and feed on the unfolding leaves, but may also damage the growing point in older plants. Larva will sometimes tunnel into older plants. The larval stage lasts about three to four weeks and the pupal stage only 10 days, so maize can be attacked by several generations. Damage is easy to spot by the ragged appearance of the leaves and the large amount of sawdust-like excrement found around the leaf whorl. Diseases and predators may greatly reduce their numbers. Liquid or granular insecticides applied to the leaf whorl are effective and should be applied before the larvae have reached 16-18 mm.
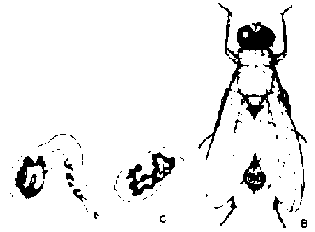
Corn earworm (Heliothis zea): A striped yellow, brown or green caterpillar. The moth deposits her eggs individually on the maize silks. Eggs are white, round, and smaller than the period at the end of this sentence, but can be easily seen with a low power magnifying glass. They hatch in three to seven days, and the larvae feed on the young silks and kernels near the ear tip. Earworms seldom interfere with pollination, since most silks become pollinated the first day they emerge from the ear. Eggs are sometimes laid on the leaves of younger plants, followed by leaf feeding in the whorl as with the armyworm. Ear damage is rarely serious enough to justify using insecticides, which would have to be applied to the silks-a time-consuming process. Varieties with long, tight husks have good resistance.

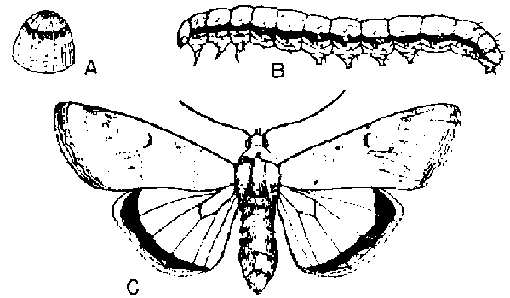
- Miscellaneous leaf-feeding caterpillars
(yellow striped armyworm, true armyworm, measuring worm, etc.): These may occasionally require foliar insecticide sprays.
Southern cornstalk borer (Diatraea), Southwestern corn borer (Zeadiatraea): Prevalent in lowland areas of Latin America. Moth larvae are about 25-mm when fully grown and are white with dark spots. Eggs are laid in overlapping rows of 10-12 on the leaves near the central veins. Eggs hatch in three to six days, and young larvae spend two to three days feeding on the leaves, making circular holes, before they bore into the stalk. Larval stage lasts several weeks, and pupation takes place inside the stalk. Control is only partly successful and requires spraying the plants during the short period before the larvae bore into the stalks or the use of systemic insecticides, some of which are very toxic.
Stalk borers (Busseola, Sesamia, Eldana, Chilo): Very common in Africa and parts of Asia and can cause serious losses. Busseola and Sesamia prefer young plants and can kill them by damaging the growing point. All four types may attack the ears on older plants in addition to the stalks. Busseola moths mate soon after emergence from the pupal stage and deposit their eggs in groups of 30-100 on the inner leaf sheath near the whorl. The larvae feed on the whorl and then tunnel into the young plant. Systemic insecticides applied to the soil or to the leaf whorl give fair to good control. Eradication of wild grasses that serve as borer hosts helps reduce numbers.
Leafhoppers (Cicadulina Dalbulus: Small, light-green, wedge-shaped insects with piercing-sucking mouth parts. Cicadulina transmits maize streak virus in Africa, and Dalbulus spreads corn stunt virus ("achaparramiento") in Latin America. Both diseases can cause serious losses. Insecticides are effective.
Grasshoppers: Cause serious losses in parts of Africa. Foliar sprays and baits are effective unless the infestation is severe.
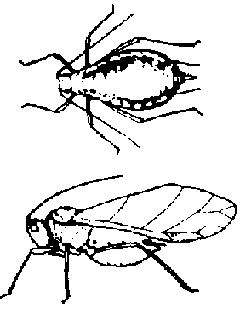
Maize aphids (Rhopalosiphum): Small, soft-bodied, green or blue, green insects that suck sap from plants and secrete a sweet substance (honeydew) on which a black mold grows. They can stunt and deform the tassels, causing poor pollination. Treatment should be considered if 50 percent of the plants have some aphids and 10-15 percent are heavily infested. Systemic insecticides give longterm control.
- Common Storage Insects of Cereal Grains
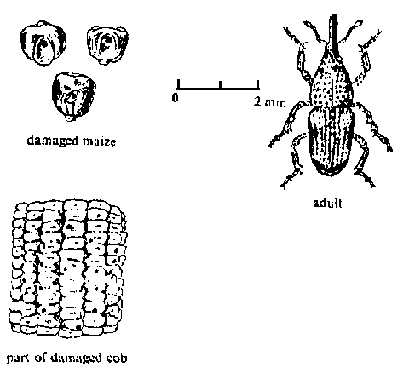
Maize weevil (Sitophilus zeamais), rice weevil (S. oryzae), and granary weevil (S. granarius): All have long snouts and are about 8.3mm long. Only the maise and rice weevils can fly and infest crops in the field. Females live several months and lay 200-400 eggs by boring holes in the kernels and depositing the eggs inside. The white, legless larvae feed on the inside of the kernels, then pupate, and finally emerge as weevils. All three species are more common in humid than dry regions.
Angoumis grain moth (Sitotroga cerealella): A small cream- or tan-colored moth with a wingspan of about 12.7 mm that is often the major stored grain pest in drier regions. Adult moths have a black fringe on the tip of each forewing. They can infest grain both in the field and during storage, but can penetrate only about the top 4-inch layer in stored, threshed grain. Maize stored as ears can be completely infested, however. Each female lays about 40-400 eggs on the outside of the kernels, and the tiny larvae burrow inside to feed. Pupation takes place inside the kernel, and the young moths emerge to begin a new cycle. The moths themselves do no feeding. Unlike most other storage insects, the angoumis grain moth can be controlled by spraying or dusting only the surface layer of stored, threshed grain with an approved insecticide like Malathion or pyrethrin.
Major Sorghum Pests
Sorghum is attacked by many of the same insects that attack maize, but two other insects can also cause serious damage.
Sorghum midge (Contarinia sorghigola: A small orange fly about 2 This is the most important sorghum pest worldwide. The adult lives only about a day and lays eggs on sorghum grain heads during flowering. Larvae hatch in two to four days and spend 9-11 days feeding on the juices of the developing seeds, preventing them from developing. The pupal stage lasts two to six days for a total life cycle of just 15-20 days.
Some local varieties show fair resistance to this pest. Sorghum heads can be sprayed with an insecticide three to five days after they emerge from the boot. Sorghum should not be planted near young sorghum or Johnsongrass, and out of season sorghum heads should be removed from fields. In cooler areas, the larvae pupate in a silken cocoon, but may also do this in very hot, dry weather. Plowing under residues may help control the pest in these cases.
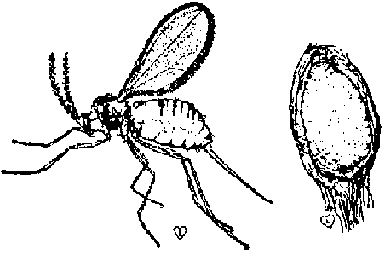
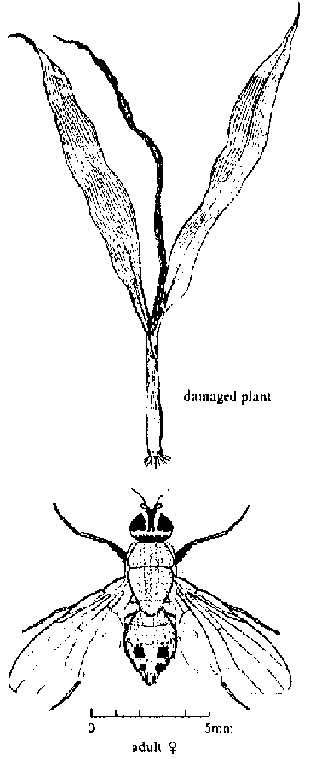
Sorghum shoot fly (Atherigona soccata): A major pest in Africa and Asia. Adults look like small houseflies and lay eggs on the leaves of young plants. Larvae move down into the leaf whori and then bore into the young stem, often killing the growing point. The youngest leaf then turns brown and withers-this condition is called "deadheart". Some sorghum varieties show shoot fly resistance. Insecticides applied to the whorl are not as effective as pre-plant applications of systemic insecticides to the soil.
Millet Pests
Millet is attacked by many of the same insects as sorghum, including the shoot fly, midge, and stem borer, but damage is usually less serious. The millet grain midge (Geromyia pennisetti) is common in the savanna region of Africa. A caterpillar (Masalia spp.) has increased in numbers in the northern savanna and Sahel during the 1970's and can cause serious head damage.
Peanut Pests
White grubs, wireworms, and rootworms attack peanut roots, and the latter two also attack the pods.
Termites can severely attack the pods, but damage is usually patchy. Treating planting seed with an insecticide, destroying the nests with Chlordane or other insecticides, or applying insecticides broadcast or banded along the crop row are effective on termites.
The lesser cornstalk borer may bore into stems and pods. In Senegal, about a dozen types of millipedes damage pods. Any pod damage increases the likelihood of aflatoxin ( a harmful toxin and carcinogen produced by Aspergillus fungus; see section on diseases).
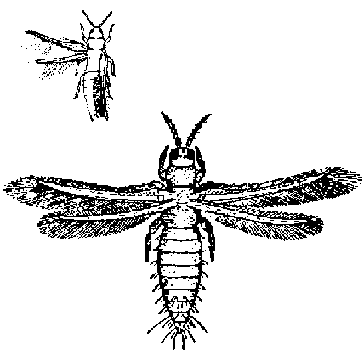
Thrips: These tiny(1 mm) yellow to black insects have two sets of fragile wings which are fringed with hairs along the rear edge. Immature thrips (nymphs) are light yellow to orange and smaller than the adults. If disturbed, thrips will jump or hop. They can cause serious damage by feeding in the buds or folded leaflets. They have rasping-sucking mouthparts which cause the leaves to be scarred and distorted as they unfold. Thrips can also spread spotted wilt virus.
Leafhoppers: Can be another major pest. Adults are around 3 mm long, pale green, and wedge-shaped. Immature leafhoppers (nymphs) are similar in appearance to adults, but smaller and without wings. Both stages have piercing-sucking mouthparts. The first signs of leafhopper damage are yellow "V" formations at the leaf tips, and severe cases can cause stunting and leaf drop.
Spider mites (Tetranychus and other species: Common in hot, dry conditions. They are sucking insects, and feeding damage may appear as translucent dots on the leaves. Some insecticides will not control mites, while Kelthane is effective only against mites.
Corn earworms (Heliothis spp.), armyworms (Spodopters, Pseudaetia), and other caterpillars feed on the leaves. Blister beetles (Epicauta spp.) are brightly colored with alternate bands of black and red or yellow-they feed on the flowers. Aphids occasionally attack peanuts. One species (Aphis croccivora) spreads rosette virus, a serious problem in Africa.
Peanuts are very susceptible to attack by storage insects. The groundnut bruchid (Caryedon spp.) is a serious pest in West Africa. This weevil lays eggs on the pods after the crop has been lifted from the ground, and the larvae tunnel into the pods and kernels.

Bean Pests
The following information is based on The International Center for Tropical Agriculture (CIAT) studies on the mayor insect pests of common beans (Phaselous vulgaris) in Latin America.
- Seedling Stage Insects
Cutworms and white grubs may cut off the stems of young seedlings. White grubs are usually only serious when beans are planted following pasture. The lesser cornstalk borer may bore into the stem just below the soil surface and move upwards and kill the plant. Clean fallowing for long periods or heavy flooding will control these borers as will granular insecticides applied near the seed row at planting.
- Leaf Feeding Insects
Many species of beetles, such as the banded cucumber beetle (Diabrotica balteata), bean leaf beetle (Cerotoma), flea beetle (Epitrix), and Mexican bean beetle (Epilachna), attack bean leaves. The most serious damage is caused during seedling stage when the insects can defoliate the plant more readily, or during flowering. Both larvae and adults of the Mexican bean beetle feed on the leaves. The larvae of the other beetles feed mainly on the roots of beans, maize, and certain weeds.
Caterpillars usually do not cause economic damage on bean leaves. The bean leafroller (Urbanus or Eudamus), saltmarsh or wooly bear caterpillar (Estigmene), and Hedylepta caterpillar are the most common.
- Sucking Insects
The leafhopper species Empoasca Kraemeri is the most serious insect pest of beans in Latin America and is also found in other regions. It does not transmit virus (some other leafhoppers do) but causes severe stunting, yellowing, and leaf curling. Work done by CIAT has shown that yields are reduced about six percent for each leafhopper present per leaf. Eggs hatch in eight to nine days and the nymphs feed on the plants for eight to eleven days before becoming adults. The adult stage lasts about 60 days and is more damaging. Beans grown with maize are less affected than pure stands. Mulching reduces leafhopper populations. Leafhopper problems are generally more severe in hot, dry weather.
Several species of aphids attack beans, although their feeding causes little direct damage, they can transmit bean common mosaic virus.
Several species of mites attack beans. The red spider mite is found on the lower leaf surface, and heavy infestations turn the leaves brown. The tarsonemid mite is too tiny to be seen without a magnifying glass, but causes young leaves to curl up-ward. Mites are seldom serious except during the dry season.
Whiteflies (Bemisia spp.) do not usually cause direct damage but can transmit bean golden mosaic virus and bean chlorotic mottle virus. They are often controlled by natural predators, and most insecticides are effective.
- Pod Borers
The bean pod weevil (Apion godmani) is a serious problem in Central America. Adults are black and about 3 mm long and they feed on flowers and pods without causing much damage. However, the female chews a small hole in young pods and deposits an egg. The larva feeds on the inner pod and the developing seeds. Pupation takes place in the pods, and the adults emerge near harvest time. Bean types vary in their resistance. A number of insecticides give good control if applied once at a week past flower initiation and again a week later. Carbafuran applied at planting gives excellent control.
Bean bruchids (Acanthoscelides obtectus and Zabrotes subfasciatus ) are snout less weevils about 2.5mm long and are the major storage pests of beans. A. obtectus predominates in cooler areas, while Z. subfasciatus prefers warmer regions. Life cycles for both are very similar with eggs being laid on stored beans or in cracks of growing pods in the field. The larvae tunnel into the seeds to feed.
Adult weevils are short-lived and do little feeding. Both types of weevils may be present initially, but A. obtactus is a better competitor at lower temperatures and will eventually predominate under these conditions. These bruchid weevils are estimated to cause storage losses of up to 35 percent in Mexico and Central America.
Slugs occasionally cause serious leaf damage and are mainly active at night or on wet, cloudy days. Damage is msot likely along field borders but may move inward. Cleaning the field of weeds and plant debris helps control them, but baits are the most effective means of control. Slime trails on the leaves indicate the presence of slugs.
- Cowpea Pests
The caterpillar Maruca testulalis is the major cowpea pest in the Savanna region of Africa. It attacks flowers, pods, and leaves, causing yield losses up to 70-80 percent.
Coreid bugs (plant bugs) are larger sucking insects that feed on green pods and cause them to shrivel and dry prematurely.
The leaf feeding beetle Ootheca mutabilis can cause yield reductions when young plants are heavily attacked. It also carries yellow mosaic virus.
The flower thrip (Megalurothrips sjostedti) is a major cowpea pest in tropical Africa. Thrips have suckingrasping mouthparts and are very small (about 1 mm or less).
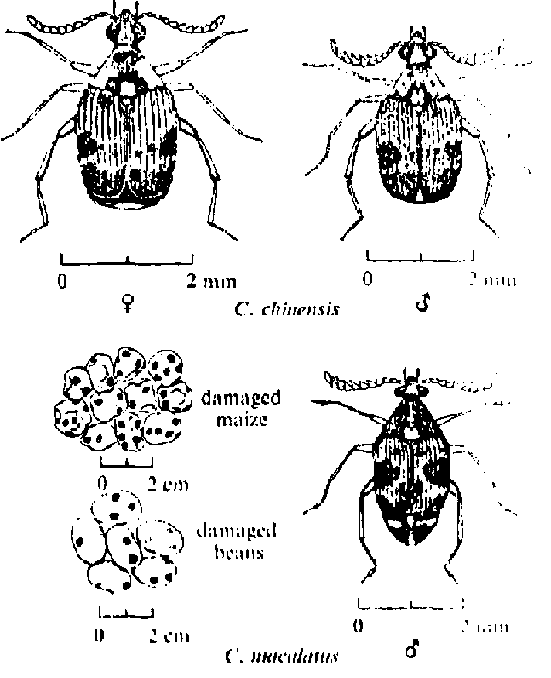
The snoutless bruchid weevils (Callosobruchusspp) infest cowpeas both in the field and in storage. The adults can fly up to a kilometer and are most likely to infest crops downwind from strong facilities. The 2.5 mm adults lay eggs on the pods or seeds, and the larvae bore into the grain.
IITA, in Nigeria, estimates that one-third of the cowpea crop in Africa is destroyed by bruchids.
Methods of insect control
Non-Chemical Methods
Many natural controls act to keep insects in balance:
- Weather factors like temperature and rainfall can restrict the distribution of an insect species. For example, mites and leafhoppers are usually more prevalent under dry conditions.
- Geographic barriers like large bodies of water, mountains, and deserts can also limit insect distribution.
- Frogs, toads, lizards, moles, and birds are some of the many animals that feed largely on insects.
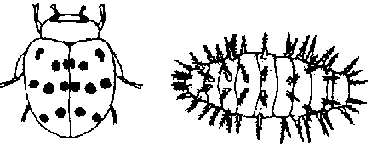
- Beneficial predator insects like ladybugs feed on aphids, while others like the braconid wasp and tachnid fly lay eggs on or in certain pests which are killed by the developing larvae. Some predator insects like the praying mantis eat beneficial insects as well, however. Insects are also attacked by viruses, fungi, and bacteria which help keep populations down.
As agricultural activities have increased, many of these natural balances have been upset and can no longer be relied upon to keep harmful insects under control. Monoculture and the existence of vast areas under cropping have led to marked increases in a number of insect pests. Indiscriminate use of pesticides has actually resulted in buildup of harmful insects in some cases. Many of the traditional crop varieties, despite their lower productivity, have better insect resistance than some of the improved varieties.
Biological Control
Biological control is the purposeful introduction of predators, parasites or diseases to combat a harmful insect species. About 120 different insects have been partially or completely controlled by this method in various parts of the world. Microbial insecticides such as Bacillus thuringiensis (effective against a few types of caterpillars) are now commonly used by farmers and gardeners in many areas. Unfortunately, biological control measures are presently effective against a very small portion of harmful insect species.
Cultural controls
Cultural controls such as crop rotation, intercropping, burying crop residues, timing the crop calendar to avoid certain insects, and controlling weeds and natural vegetation that harbor insects are all effective control methods for some insects. In most cases, however, cultural controls need to be supplemented by other methods.
Varietal Resistance
Crop varieties differ considerably in their resistance to certain insects. For example, maize varieties with long, tight husks show good resistance to earworms and weevils. Researchers at CIAT have found that some bean varieties are relatively unaffected by leafhopper damage during the wet season, while others suffer yield losses of up to 40 percent. Screening for insect resistance is an important part of crop breeding programs.
"Organic" Controls
"Organic" control refers to non-chemical methods in general. These include the application of homemade "natural" sprays made from garlic, pepper, onions, soap, salt, etc., and the use of materials like beer to kill slugs and wood ashes to deter cutworms and other insects. Some of these "alternative" insecticides are slightly to fairly effective on small areas like home gardens and where insect populations are relatively low. They are seldom feasible or effective on larger plots, especially under tropical conditions that favor insect buildup.
Chemical Control
Chemical control refers to the use of commercial insecticides in the form of sprays, dusts, granules, baits, fumigants, and seed treatments. While some of these insecticides like rotenone and pyrethrin, are naturally derived, most are synthetic organic compounds that have been developed through research.
Advantages of Insecticides:
- They act rapidly.
- They are the only practical means of control once an insect population reaches the economic threshold of damage on a commercial-size plot.
- They are available with a wide range of properties, species effectiveness, and application methods.
- They are relatively inexpensive, and their proper usage can often return .00 5.00 for every .00 spent.
Disadvantages of Insecticides:
- Insect resistance to pesticides: This is a growing problem. By 1961, 60-70 species had developed resistance to certain products, and the number had increased to around 200 by the mid-1970's.
- Outbreaks of secondary pests: Few insecticides kill all types of insects, and some actually promote the increase of certain pests. For example, continual use of Sevin (carbaryl) in the same field may increase problems with some types of aphids which it does not control well.
- Damage to non-target species: These include beneficial predators such as bees and wildlife.
- Residue hazards: Some chlorinated hydrocarbon compounds like DDT, Aldrin, Endrin, Dieldrin, and Heptachlor are highly persistent in the environment and may accumulate in the fatty tissues of wildlife, livestock, and humans. Many other insecticides are broken down into harmless compounds fairly rapidly.
- Immediate toxicity: Some insecticides are extremely toxic in small amounts to humans and animals. Again, it is important to realize that insecticides vary greatly in their toxicity.
Current Status of Insecticide Use in the Reference Crops
At the present time and for the immediate future, insecticide usage will often be an essential part of any package of improved practices for the reference crops. For this reason, all extension workers must learn the basic principles of safe and effective insecticide application. Some extension workers may be personally opposed to the use of these chemicals, but it is a fact that farmers throughout the developing world are using them, often in an unsafe and indiscriminate manner due to the lack of proper instruction. Most developing countries have few, if any, pesticide regulations or restrictions on environmentally harmful products like Aldrin or highly toxic ones like Parathion. By instructing farmers in safety precautions and in the appropriate choice and use of insecticides, the incidence of human poisoning and possible environmental damage can be greatly reduced.
Integrated Pest Control
The disadvantages of total reliance on insecticides have given rise to integrated pest control or pest management which involves the judicious use of these chemicals based on the following guidelines and principles:
- The development and use of cultural and other nonchemical control methods to avoid or reduce insect problems.
- Determining crop tolerance to pest damage based on the principle that complete freedom from pests is seldom necessary for high yields. Nearly all plants can tolerate a surprising amount of leaf loss before yields are seriously affected.
- The appropriate timing and frequency of treatments to replace routine, preventative spraying. Treatments are not initiated before the particular insect has reached the economic damage threshold, which will vary considerably with the species. Insect scoutinglooking for related kinds and number of insects and their density and population counts-is an essential part of this system.
The advent of integrated pest control dates back to the early 1970's, and most of the efforts have been directed at cotton where insecticides frequently account for up to 80 percent of total production costs. Some remarkable successes have been achieved with other crops as well. For the reference crops, integrated pest control is still in the very early stage, especially in developing countries.
USING INSECTICIDES SAFELY
Insecticide safety guidelines, toxicity data, and first aid measures are covered in Appendix J, which should be referred to before working with insecticides.
Some important facts on insecticides
Pesticide Terminology
Pesticide: A general term referring to chemicals that control crop insects, mites, weeds, diseases, nematodes and rats.
Miticide (acaricide): A pesticide that kills mites. Mites are related to spiders and not all insecticides will kill them. Some pesticides like Kelthane control only mites, while others like Diazinon and Malathion kill mites and other insects.
Nematocide: A pesticide that kills nematodes. A few insecticides like carbofuran and Mocap will also control nematodes, but most will not. Some nematocides like Nemagon control only nematodes, while others like VAPAM, Basamid, and methyl bromide are general soil sterilants that kill insects, weeds, fungus, and bacteria as well.
Systemic vs. Non-Systemic Insecticides
Nearly all modern insecticides are contact poisons that kill insects by being absorbed through their bodies. Contact poisons act as stomach poisons if eaten by insects. Most insecticides are non-systemic and are not absorbed into the plant. Systemic insecticides are absorbed into the plant sap, and most are translocated through-out the plant. Most systemic insecticides like Metasystox, Dimethoate (Rogor, Perfecthion), and Lannate are sprayed on plant foliage. Others like carbofuran, Thimet, and Disyston, are applied to the soil in a band along the crop row, where they are absorbed by the plant roots and then translocated to the stems and leaves. Some of these soil-applied systemics will also control certain soil insects.
There are several considerations in choosing between a systemic and nonsystemic insecticide:
- Systemic insecticides are especially effective against sucking insects like aphids, leafhoppers, stinkbugs, and thrips since these feed on the plant sap. However, many non-systemic contact insecticides will also control sucking insects adequately.
- Most systemics are less effective against caterpillars and beetles, but may give good control of some stem borers.
- Foliar-applied systemics may remain in the plant for up to three weeks. Soil-applied systemics may provide control for up to six weeks. However, this also means that they must not be applied close enough to harvest time to cause residue problems.
- Most systemics will not harm beneficial insects.
- Foliar-applied systemics are not broken down by sunlight or washed off the leaves by rainfall as with non-systemics.
- Since they are translocated, systemics do not require uniform spray coverage when they are applied to the leaves. New growth occuring after application is also protected.
- Some systemics like Thimet, Di-syston, and Systox are highly toxic both orally and dermally. However, the same is also true with some non-systemics like Parathion and Endrin. (See Appendix J.)
Types of Pesticide Formulations
Most insecticides are available in several types of formulations:
- WETTABLE POWDERS SOLUBLE POWDERS: These range in strength from 25-95 percent active ingredient and are meant to be diluted with water and applied with a sprayer. For example, Sevin 50 W is a wettable powder containing 50 percent pure carbaryl by weight. Once mixed with water, wettable powders require periodic agitation (shaking or stirring to keep them from settling to the bottom. Soluble ' powders ("SP") are completely soluble and do not require agitation.
- EMULSIFIABLE CONCENTRATES ("EC" or "E"): these are high strength liquid formulations. Like wettable powders, EC's are meant to be diluted with water and applied with a sprayer. They contain 20-75 percent active ingredient. In countries using pounds and gallons, a label that reads "Malathion 5 E" would refer to a liquid formulation of malathion that contains 5 1bs. active ingredient per gallon. Where liters and grams are used, EC's are often labeled in terms of grams of active ingredient per liter. For example, Tamaron 600 is a liquid formulation of Tamaron containing 600 grams of active ingredient per liter.
- DUSTS ("D"): Unlike WP's and EC's, dusts are low strength formulations (1-5 percent active ingredient) and are meant to be applied without dilution by a duster. Dusts are usually more expensive than WP's or EC's due to higher transport costs per unit of active ingredient. However, if dusts are blended within the country, they may be competitive cost-wise and are especially suited to situations where a farmer has difficulty transporting water to his field. They do not stick to the leaves as well as sprays and are more easily washed off by rainfall. Retention is improved if they are applied while the leaves have dew on them. Dusts pose more of an inhalation hazard than sprays. They should never be mixed with water.
- GRANULES "(G"): Like dusts, granules are low-strength formulations meant to be applied without dilution. They are especially well suited for soil applications and for placement in the leaf whorls of maize and sorghum to control armyworms. Granules cannot be effectively applied to leaves, because they roll off. Furadan 3G is a granular formulation that contains 3 percent pure carbofuran.
- FUMIGANTS: These are available as pellets, granules, liquids, and gasses whose fumes kill pests. They are used to kill insects in stored grain or applied to the soil to kill insects, nematodes, and other pests.
- BAITS: These are usually the most effective formulations for controlling cutworms, crickets, slugs, and snails.
Cutworms are most effectively controlled with baits rather than with sprays. Baits should be scattered near the plants in the late afternoon if rainfall is unlikely. Bait should not be left in clumps which might poison birds or livestock. One kg of bait should cover about 400 sq. meters.
Cutworm bait recipe:
25 kg of carrier (sawdust, rice bran, maize flour, etc.)
3 1 of molasses
1 - 1.25 kg active ingredient of trichlorfon or carbaryl
Water can be added to moisten the bait.
Slugs and snails can be controlled by applying baits in the late afternoon in a band along the field's borders or within problem areas. It should not be applied if rain is expected that night, since rain may wash the insecticide from the bait.
Slug and snail bait recipe:
25 kg maize flour or bran
10 1 molasses
65 g metaldehyde (a stomach poison of lowdermal toxicity) or 0.5 kg active ingredient trichlorofon or 0.5 kg active ingredient carbaryl
Chemical Classes Of Insecticides
Commercial insecticices fall into three main chemical classes or groups:
- Chlorinated hydrocarbons (organochlorines): Many of the insecticides in this group such as DDT, Aldrin, Endrin, and Dieldrin have very long residual lives and have caused environmental problems such as fish kills. However, other members such as Methoxychlor are readily biodegradable. Toxicity to humans and animals varies greatly within this group (see Appendix K).
- Organic Phospates (organophosphates): The insecticides of this group such as Malathion, Dipterex, Diazinon, and Parathion have a much shorter residual life than most of the organochlorines. Their toxicity to animals and humans varies greatly. Some like Parathion, TEPP, Endrin, and Thimet are highly dangerous, while others like Malathion, Gardona, and Actellic are among the safest insecticides available.
- Carbamates: Relatively few insecticides belong to this group and they tend to be of moderate to low toxicity. The exceptions are carbofuran and methomyl which have very high oral toxicities. Carbaryl and propoxur are probably the bestknown carbamates. The residual life of this group varies from short to moderate.
Insecticide Dosage Calculations
For all types of pesticides, there are four basic ways of stating dosages:
1. Amount of active ingredient (pure chemical) needed per hectare or acre.
2. Amount of actual formulation (i.e. Sevin 50 WP or Furadan 3 G, etc.) needed per hectare or acre.
3. Amount of actual formulation needed per liter or gallon of water.
4. As a percentage concentration in the spray water.
Types 1 and 2 dosages are suited more to large plots or to those pesticides (especially herbicides) needing very accurate dosage application. Sprayer calibration is needed in both cases to determine how much water to use and how much pesticide to add to each tankful.
Types 3 and 4 are very general recommendations best suited to smaller plots or where dosage accuracy is not critical.
1. AMOUNT OF ACTIVE INGREDIENT NEEDED PER HECTARE: For example, a dosage might be given as 2 kg active ingredient carbaryl per hectare. This means 2 kg of pure (100%) Sevin. Since actual pesticide formulations vary in strength from l percent up to 95 percent, it takes some math to figure out how much of a given formulation is needed to supply a given amount of active ingredient. If the local agricultural supply store sells carbaryl 50 percent WP, the farmer would need 4 kg for each hectare in order to supply 2 kg active ingredient. Note that nothing is said about how much water the farmer should mix with the pesticide when he sprays it on the plants. This will depend on plant size, plant density, and the degree of coverage desired. The only way to find out how much water is needed is to calibrate the sprayer.
2. AMOUNT OF ACTUAL FORMULATION NEEDED PER HECTARE OR ACRE: A recommendation calling for 4 1 of Malathion 50 percent per hectare, for example, is somewhat simpler than Type l since it is given in terms of actual formulation rather than active ingredient. However, the farmer still needs to know how much formulation he needs for his field's area and how much water it will take to provide adequate coverage with his sprayer. This requires sprayer calibration.
3. AMOUNT OF ACTUAL FORMULATION NEEDED PER LITER OR GALLON OF WATER: If the recommendation is expressed as for example, 5 cc of Malathion 50 percent EC per 1 of water, no sprayer calibration or dosage calculation is needed. The drawback is that the amount of pesticide the farmer actually applies on his field depends entirely on how fast he or she walks while spraying, how coarse or fine the spray is, and how much pressure is used. However, if proper guidelines are followed, Type 3 recommendations are precise enough for most conditions and are the most feasible for small farmers. They should not be used for most herbicides where accuracy of dosage is critical.
4. AS A PERCENTAGE CONCENTRATION IN THE SPRAY WATER: This is basically the same as Type 3, except that the concentration of pesticide in the spray water is given in terms of percent rather than cc/liter. Such recommendations are usually based on percentage by weight, although sometimes a volume basis is used when dealing with Et's (the actual differences are slight). The percentage figure given may refer to active ingredient or to actual formulation. As with
Type 3 recommendations, no sprayer calibration is needed, and dosage accuracy is not as good as with Types 1 and 2.
Pesticide Math
Converting recommendations from an active ingredient basis to an actual formulation basis.
Once you know how much actual formulation is needed per hectare or acre, you can easily calculate how much is needed for farmers' fields by multiplying the field size in hectares times the dosage per hectare.
Following a percentage strength spray recommendation:
Determine first whether the spray's percentage strength is to be calculated in terms of active ingredient or in terms of actual formulation. For example, one recommendation might be expressed as 2 percent strength spray in terms of pure Malathion.
Another recommendation might call for using a 0.1 percent strength spray of Lebaycid 50 percent EC for controlling thrips on peanuts.
- For wettable powders When using WP's, a percentage strength spray is based on weight of pesticide to weight of water. Since 1 liter of water weighs 1 kg, these formulas can be used:
Active ingredient basis
Grams of wettable powder needed per liter of water [2% x 1000]/40%= 20/0.4 = 50g
Actual product basis
Grams of wettable powder needed per liter of water
= % strength spray desired x 1000
- For liquids (EC's)
Active ingredient basis
cc (ml) of EC needed per liter of water
= [ % strength spray desired x 1000] / % active ingredients in the EC
Guidelines for applying insecticides
When is Treatment Necessary?
Farmers should apply insecticides in response to actual insect problems rather than on a routine and indiscriminate basis. Ideally, insecticides should be used only when damage has reached the economic threshold. This level varies with the insect species, the crop, and the type and extent of damage.
General guidelines (see also the unit on major reference crop insects):
- Soil insects. These pests should be treated preventatively by making pre-planting or atplanting insecticide applications if a known problem exists. Treatments after planting are generally not effective except in the case of cutworm baits.
- Leaf-eating insects (beetles, caterpillars): Crops can tolerate considerable defoliation as long as new leaves are being continually produced. Loss of leaf area becomes more serious as the vegetative stage nears its end, although defoliation in the very late stages of grain development will not have a big effect on yield. Stem-borers usually cause more serious damage at much lower populations than most leafeating insects. The sorghum shoot fly, sorghum midge, and one species of bean leafhopper (Empoasca kraemeri) are other examples of insects that reach the economic threshold of damage at relatively low populations.
- Sucking insects: Not all species of aphids and leafhoppers spread virus diseases. For example, CIAT found that bean yields were reduced about 6 percent for each Empoasca kraemeri leafhopper present per leaf, even though this species does not transmit any viruses. Bean plants can tolerate aphids well unless they are of a species capable of transmitting common bean mosaic virus.
Using a Sprayer Effectively
Achieving the Correct Coverage
The extent and uniformity of coverage needed depend on the insects' location and whether or not a systemic insecticide is being used. In some cases such as armyworms feeding in the maize leaf whorl, the insect is very localized, so general coverage is not needed. Other insects are more general feeders and require thorough spray coverage over the whole plant. Since they are translocated, systemic insecticides do not require the uniform coverage nonsystemics do.
The amount of water need for adequate coverage varies with plant size, density, type of product (systemics versus nonsystemic), and insect location, but there are some rough guidelines:
Water rates for insecticides: When covering the entire foliage of full size plants, at least 500-550 l of water per hectare will be needed when using conventional sprayers. When spraying is localized or plants are very small, water volume may be only one-quarter of this amount.
Too much spray is being applied if there is a visible amount of runoff from the leaves, although this can also be caused by not using enough wetting agent (spreader).
Using a Spreader And Sticker
A spreader (wetting agent) reduces the surface tension of spray droplets, allowing them to spread out rather than remaining as individual globules on the leaf surface. Spreaders markedly improve the uniformity of spray coverage and also help prevent droplets from rolling off the leaves.
A sticker (adherent) is a gluelike substance that helps the spray stick to the leaf surface and resist being washed off by rainfall or sprinkler irrigation.
Many commercial stickers and spreaders are available, including combination sticker-spreaders. The pesticide label will indicate if a spreader or a sticker is needed. If spraying the soil, neither a spreader nor a sticker is needed. When spraying the leaf whorl of maize, a spreader is not needed, though a sticker might be helpful. Use of a sticker and spreader is especially important when applying most foliar fungicides.
Commercial stickers and spreaders are relatively cheap. However, if not available commercially, they can be made at home. Egg white, cassava (yuca, manioc) flour, and corn starch can be used as stickers at about 15 cc per 15 liters. Liquid dishwashing detergent makes a satisfactory spreader at about the same rate.
Non-ionic spreaders: Paraquat and diquat post-emergence herbicides are unusual in that they require the use of special non-ionic spreaders in order to avoid deactivation (loss of effectiveness). Ortho-77 is one commonly available non-ionic spreader.
Choosing a Spray Nozzle
Spray nozzles are available in a wide variety differing in output, spray pattern angle, and type of spray pattern. Proper nozzle selection has an important influence on pesticide effectiveness.
Nozzle Output: Many backpack (knapsack) sprayers come equipped with adjustable nozzles which allow the farmer to vary the output by making the spray finer or coarser. This would seem to be an advantage, buth such nozzles usually do not maintain their setting well and output can change considerably during application. This is unsatisfactory where accurate dosages are necessary, and it makes sprayer calibration difficult. Fixed orifice nozzles are available in a wide range of outputs and should be used whenever possible.
Illustration courtesy of Rohm & Hass Co., Philadelphia, Pennsylvania
Ideal tractor spray boom arrangement for applying insecticides and fungicides and achieving uniform coverage. Note that the drop nozzles are angled about 30° upward as well as 30° forward. Only one tier of "drop" nozzles may be needed on small- to medium-size crop plants.
Spray Pattern Angle: See flat spray
Type of Spray Pattern: Care should be taken to choose the right spray pattern for the job.
- Flat (Fan) Spray Nozzles are ideal for making broadcast (full coverage) applications of insecticides or herbicides over the soil surface (and small weeds). The application rate decreases at both edges, so the spray patterns of adjacent nozzles should be overlapped about three to four fingers width at the soil surface to achieve even distribution. Fan nozzles do not provide as good a coverage as cone nozzles when used to spray crop foliage. Fan nozzles are available in several different angles of spray width. Wider angles allow the spray boom to be carried closer to the ground and this lessens spray drift problems on windy days.
- Even Flat (Fan) Spray Nozzles should be used for making band applications of pesticides to the soil. Spray output does not decrease at the edges, so spray patterns should not be overlapped and used for broadcast applications.
- Solid Cone Spray Nozzles provide better coverage of plant foliage than fan nozzles but should not be used to apply herbicides and insecticides to the soil.
- Hollow Cone Spray Nozzles offer somewhat better foliar coverage than solid cone nozzles due to greater leaf agitation as the spray pattern passes over the plants.
- Whirlchamber (nonclog) Spray Nozzles are special wide angle hollow cone nozzles that can be used in place of fan nozzles. Their design reduces clogging, and drift is minimized because of the wide angle pattern (enabling lower boom height) and larger droplet size.
Nozzle Screens: Nozzles used on tractor boom sprayers usually have mesh or slotted strainers to help prevent clogging. Some backpack sprayers have strainers or can have them added on. Routine cleaning is required, especially when wettable powders are used.
Tips on Using Backpack Sprayers to Apply Insecticides
- Use good pressure and a fine spray. Pressure is too high if excessive spray drift (misting) occurs.
- Maintain a steady pace through the field. Avoid pausing at each plant unless the crop is very large.
- Rotate your wrist while spraying so that the spray hits the foliage from different angles.
- Keep the nozzle far enough away from the foliage so that the spray has a chance to spread out before hitting the leaves.
- If using a wettable powder, remember to periodically shake the sprayer to keep the pesticide in solution.
- Keep a piece of soft wire handy for cleaning out clogged nozzles, but use it gently to avoid damaging the nozzle opening.
- Do not spray plants when their leaves are wet or when rain is likely within a few hours afterwards.
- Do not add wettable powders or EC's directly to the sprayer tank. First mix them thoroughly in a bucket with several liters of water. Make sure wettable powders are completely dissolved.
Pesticide Compatibility
Most pesticides are compatible with each other in the spray tank, but check the fable to make sure. In some crops like peanuts and vegetables, foliar insecticides and fungicides are often applied together. Spray compatibility charts are available from many pesticide companies.
Water with a pH of 8.0 or above (alkaline) causes a rapid breakdown of organic phosphate insecticides. Such high pH water is usually confined to limestone or low rainfall areas. Special buffering agents are available to lower the pH if necessary.
Certain insecticides are phytotoxic (injurious) to certain crops. Always check the label instructions. Wettable powder formulations tend to be less phytotoxic than emulsifiable concentrates, especially in temperatures over 32 C.
|
Sorghum: |
Trichlorfon causes severe injury. Azodrin and methyl parathion cause some injury. |
|
Peanuts: |
Minor foliar injury which shows up as reddish brown spots on the earliest leaves is sometimes caused by soil applications of carbofuran, Thimet, and Disyston. The plants usually outgrow the damage with no yield reduction. Runner varieties on sandy soils are the most sensitive, and dosage should be reduced by 25 percent under these conditions. |
Insecticide Recommendations For the Reference Crops
Particular pesticides are not recommended for the reference crops in this manual because of the potential misclassification of pest problems and misused pesticides. Rather than rely on this manual for pest diagnosis and pesticide selection, it is recommended that you rely on the insecticide recommendations of your country's extension service if they are known to be effective and if they do not involve the use of high-toxicity Class 1 chemicals (see Appendix K). Before using any insecticide, refer to the safety guidelines and toxicity data in Appendix K. Always know the relative toxicity and environmental hazards of the products you use or recommend.
Disease control
Types of Diseases And Their Identification
Parasitic versus Non-parasitic Diseases
Parasitic diseases are caused by certain types of fungi, bacteria, and viruses that invade plants and multiply within their tissues.
Non-parasitic (non-infectious) diseases are caused by unfavorable growing conditions or other nonparasitic factors such as:
- Excesses, deficiencies or imbalances of soil nutrients
- Excessive soil acidity or alkalinity
- Temperature extremes
- Poor drainage or drought
- Mechanical, fertilizer or pesticide injury
- Air pollutants like ozone and sulfur dioxide.
Some of these non-parasitic conditions produce symptoms that can be confused easily with those of parasitic diseases.
Fungal Diseases
Fungi are actually tiny parasitic plants without roots, leaves or chlorophyll which feed on living or decaying organic matter. They reproduce and spread by means of microscopic seeds called spores. Some fungi, such as those that help break down crop residues into humus, are beneficial. Fungi can penetrate directly into seed, leaf or rock tissue or can enter through wounds or natural openings. General types of fungal diseases are leaf spots leading to possible defoliation; rotting of seeds, stems, stalks, roots, grain heads, pods, and ears; and storage molds and wilts.
Diseases caused by fungi are by far the most common diseases of the reference crops because the spores are highly resistant to unfavorable conditions. They are spread easily by wind, water, soil, and farm implements, and some types can also be carried by the crop seeds themselves. Most fungal diseases develop and spread much more readily under high humidity and moisture. An important and common characteristic of fungal diseases is their ability to mutate to produce new races that are resistant to certain fungicides.
Bacterial Diseases
Bacteria are microscopic single cell organisms that multiply by cell divisor. Like the fungi, some bacteria are beneficial and perform essential functions like converting unavailable organic forms of soil nutrients to available inorganic (mineral) forms. Others invade plants and cause diseases that produce leaf spots, wilts, galls, and fruit and stem rots. For several reasons, bacterial diseases are generally much less prevalent than fungal diseases.
- Bacteria lack a resistant spore stage and are very dependent on favorable temperature and moisture conditions.
- Unlike the fungi, bacteria cannot forcibly penetrate into plant tissue but must enter through natural openings or wounds.
- Although bacterial diseases can be spread by wind-driven rain, field equipment, and certain types of insects (mainly some beetles), they are transmitted much less rapidly than fungal diseases.
Viral Diseases
Viruses are microscopic particles consisting of a core of nucleic acid (genetic material) surrounded by a protein coat. Viruses can multiply by diverting living host cells into the production of more virus particles and can also mutate to produce different strains. They are largely spread by sucking insects such as aphids, leafhoppers, and thrips. The relationship between these insect vectors (insect that transmit disease) and the viruses is sometimes very specific. For example, peanut rosette virus is transmitted by only one species of Aphid. Weeds are susceptible to certain viruses and serve as alternate hosts for viral diseases which are transmitted by sucking insects to crops.
Viruses usually do not kill plants, but can greatly reduce yields and quality. A wide variety of symptoms are produced such as leaf mottling (blotching), leaf curling, chlorotic (yellow) or necrotic (dead) spots on the leaves, leaf striping, and excessive branching.
How to Identify Plant Diseases
Some plant diseases can be identified readily by nonprofessionals right in the field. In other cases, however, accurate diagnosis requires a good deal of field experience or even the expertise of a trained plant pathologist and lab facilities. For more information on identifying plant diseases, see Appendix I, "Troubleshooting Common Crop Problems." Resources that give detailed descriptions of diseases of the reference crops can be found in the bibliography.
Methods of Disease Control and Effectiveness
Prevention versus Cure
Most diseases such as viruses and the bacterial and fungal rots of seeds, seedlings, roots, stalks, and stems cannot be controlled once they enter plant tissue. Fair to good control of fungal leafspots can be achieved with foliar fungicides but this is usually uneconomical with low value crops like maize, millet, and sorghum. Disease control methods are therefore geared much more toward prevention rather than cure.
Non-Chemical Disease Control Methods
- Resistant varieties: Disease resistance is a top priority among plant breeders. Breeders have located genetic sources of resistance to some of the more serious diseases, especially viruses and other types that lack effective or economical chemical control measures. However, resistance does not mean 100 percent immunity, and the ability of viruses and fungi to mutate into new races has posed some problems.
- Disease-free seed: Some diseases like bacterial blight and common mosaic virus of beans can be carried by the seeds. The use of certified seed that is disease-free is an important management practice in many bean-growing areas.
- Controlling host plants and insect vectors: This is especially important for controlling certain viral diseases and involves the removal of host weeds and other natural vegetation that serve as sources of infection. In some cases, non-susceptible barrier crops are planted around a field in a 15-20 m wide strip to "decontaminate" sucking insects before they reach the susceptible crop. (Usually not practical for the small farmer). Also included is the roguing (removal) of diseased crop plants attacked by viruses. However, roguing is not effective for most fungal and bacterial diseases.
- Crop residue management: The burning or plowing under of crop residues is an effective prevention method for a few diseases like Southern stem rot of peanuts.
- Other management practices: Several of these may help minimize certain disease problems: not cultivating plants while they are wet; avoiding crop injury at or before harvest; irrigating in the morning when sprinklers or hand watering are used so that crop leaves are dry at night; using raised beds to improve drainage and disinfecting tools.
- Crop rotation: This can reduce the incidence of many fungal and bacterial diseases, especially those that are soil~ borne, but will have little effect on viruses. There is nothing wrong with monoculture from a disease standpoint as long as resistant varieties are being continually developed and introduced in response to new problems. However, this is unlikely in the developing countries.
- Intercropping: This practice may reduce or intensify disease problems, depending on the crop mixtures involved and whether they share some diseases in common.
Chemical Disease Control Methods
- Fungicides can be applied to seeds, the soil, and crop leaves and will provide fair to good control of certain fungal diseases. They are mainly applied as protectants.
Seed treatment with a fungicide dust or liquid will effectively prevent seed rots (pre-emergence "damping off") caused by soil fungi. This method will also kill any fungal diseases borne on the seedcoat surface such as loose smut and covered smut which attack adult sorghum plants.
Since seed treatments mainly protect the seed, they are not as effective at preventing seedling blights (rots) and seedling root rots. A systematic seed treatment fungicide called Vitavax (Carboxin) gives somewhat better control.
Seed treatments will not control any soil-borne or airborne fungal diseases that attack older plants such as leaf spots, stalk rots, stem rots, and root rots.
Fungicide applications to the soil are sometimes helpful. Some fungicides like PCNB (Terrachlor), Vitavax (Carboxin), and Benlate (benomyl) can be applied as sprays or dusts to the seed furrow or to the row during crop growth to control certain fungal stem and root rots.
Such soil applications are seldom necessary or economical for maize, sorghum, and millet, but can be profitable on high~yielding peanut and bean crops where disease problems exist.
Foliar fungicides can be applied as dusts or sprays to crop foliage to control fungal leaf spot diseases. Most foliar fungicides act as protectants to help prevent the occurrence or spread of leaf spots. Some of the recently developed systemic fungicides like Benlate (benomyl) and Mertect (Thiabendazole) also have erradicant properties.
Most foliar fungicides have little or no effect on bacterial leaf spots, but copper base fungicides provide fair to good control.
Foliar fungicides are usually-not economical for maize, sorghum, and millet,but are often essential for control of Cercospora leaf spot in peanuts and can be very profitable in this case. Their use on beans may be justified where yields are in the medium to high range and fungal leaf spots become serious.
- Soil sterilants like methyl bromide, formaldehyde, Basamid, and Vapam will control soil fungi, bacteria, insects, weeds, and nematodes. They are applied in advance of planting and allowed to disperse before the seeds are sown. Soil sterilants are frequently used on seedbeds for growing tobacco and vegetable transplants, but are too expensive for use with the reference crops.
- Antibiotics like Streptomycin and Terramycin are bactericides used as foliar sprays or transplant dips to control certain bacterial diseases. Other antibiotics like Kamusin (Kasugamycin) and Blasticidin are effective against certain fungal diseases such as rice blast, and are widely used in Japan. Their high cost makes them uneconomical for use on the reference crops. There are several problems associated with antibiotics, namely residues, the development of resistant races of fungi and bacteria, and occasional phytotoxicity.
- Use of insecticides to control insect vectors: Is seldom completely effective since 100 percent control is impossible.
Integrated Disease Control
Integrated disease control involves the combined use of nonchemical and chemical methods. Except for the mercury base fungicides sometimes used as seed dressings, the fungicides pose few toxic or environmental threats, unlike some insecticides. The incentive for integrated disease control is based on economics and the fact that many diseases cannot be controlled adequately with chemicals.
Major diseases of the reference crops
Maize
Maize Fungal Diseases
Seed Rots and Seedling Blights
These are often referred to as pre-emergence and post-emergence "damping off" and are caused by soilor seed-borne fungi. Seeds may be killed before germination or seedlings may be destroyed before or after they emerge from the ground. Damping off is most prevalent in cold, poorly-drained soils and with damaged seed (cracked seedcoat, etc.). Problems are less likely where conditions favor rapid germination and emergence (i.e. warm weather, adequate soil moisture). Symptoms: Above-ground signs are yellowing, wilting, and death of the seedling leaves, but this can be confused easily with injury by wind, wind-blown sand, fertilizers, herbicides, and insects. Examine the below-ground portion of the plants and look for rotted seeds, soft rot of the stems near the soil surface, and rotted, discolored roots. Control: Use good quality seed, free of molds and damage, that has been treated with a fungicide like Captan or Arasan (thiram) for protection during germination. Seed treatment is mainly effective against seed rot.
Helminthosporium Leaf Blights
Several species of Helminthosporium fungi attack maize leaves, but the two most important are H. Maydis (Southern leaf blight) and H. turcicum (Northern leaf blight). Helminthosporium maydis is more prevalent in hot, humid areas, but both species can occur on the same plant. Symptoms of H. Maydis: There are two major races of H. maydis and they have different symptoms. Race "O" leaf spots are small and diamond~shaped when young and then elongate to about 2-3 cm and may grow together, killing large areas of leaf. Race "T" leaf spots are oval and larger than those of race "O" and attack the husks and leaf sheaths, unlike race "O". Maize hybrids utilizing "Texas" male sterile cytoplasm (genetic material) in their production are very susceptible to race "T". This became evident during the severe and unexpected outbreak of H. maydis race "T" in the U.S. Corn Belt in 1970. Most hybrids now utilize "N" male sterile cytoplasm in their production to overcome this problem. Symptoms of H. turcicum: Northern leaf blight prefers high humidity and low temperatures. Small, slightly oval, water-soaked spots first appear on the lower leaves and eventually become rectangular in shape and grow to a length of 2.5-15 cm. These lesions are grayish-green to tan and can cause severe defoliation. Control: Resistant varieties offer the best protection. Seed treatment with a fungicide is of no help. Foliar fungicides give fair to good control but are seldom economical since they must be applied every 7-10 days,
Maize Rusts
Three types of rust attack maize: common rust (Puccinia sorghi), Southern rust (Puccinia polysora), and tropical rust (Physopella zeae).
Common rust occurs more frequently in cool, moist weather and produces small, powdery, cinnamonbrown pustules on both surfaces of the leaves. Southern rust is more common in warm humid regions and produces smaller, lighter-colored pustules than common rust. Tropical rust is confined to the tropical regions of Latin America and the Caribbean. The pustules vary in shape from oval to round and occur beneath the leaf epidermis (outer layer). They are cream colored and very small and are sometimes surrounded by a black area. Control: Resistant varieties are the best protection. Fungicide sprays are seldom economical.
Maize Downy Mildews
At least nine species of Sclerospora (Sclerophthora) fungi attack maize. At present, they are mainly confined to parts of Asia and Africa, but also appear to be spreading throughout the Americas. Symptoms vary with the species, age of plants when infected, and the climate, but usually include chlorotic striping of the leaves and leaf sheaths, stunting, excessive tillering, and deformities of the ears and tassels. A downy growth (mildew) may occur on the undersides of the leaves in later stages. Some of these symptoms may be confused with viruses.
Some of the more common downy dildews are listed below with their control measures: Crazy Top (S. macrospora): Rare in the true tropics but of world-wide distribution in temperate and warm-temperate climates. Crazy top causes the tassel to mutate into a mass of leafy bunches and is provoked by one or more days of flooding before seedlings have reached the four to five leaf stage. Adequate soil drainage is the only control.
Sorghum Downy Mildew (S. Sorghi): Widespread. Controls: Using resistant varieties, removing and destroying infected plants, and avoiding maizesorghum rotations in infected fields. Green Ear Disease or Graminicola Downy Mildew (S. graminicola): Occurs on various grasses but is usually not important in maize. Sugarcane Downy Mildew (S. sacchari): Mainly confined to Asia and the South Pacific. Controls: Eliminating the disease by using healthy planting material, growing maize in areas free of the disease and where sugarcane is not grown extensively, removing and destroying infected plants, and using resistant varieties. Fungicide sprays are used in some areas. Philippine Downy Mildew (S. philippinensis): This is the most important maize disease in the Philippines and also occurs in Nepal, India, and Indonesia. Controls: Removing and destroying infected plants, using resistant varieties and fungicide sprays where economical.
Common Smut and Head Smut
Common smut (Ustilago maydis): A fungus that causes galls (swollen areas on plant tissue) 1520 cm in size which form on any above-ground part of the plant. When young, the galls are shiny and whitish with soft interiors, but later turn into a mass of black, powdery spores. Early infection can kill young plants, but common smut is seldom a serious problem. Controls: Using resistant varieties and avoiding mechanical injury to plants. Good soil fertility is helpful. Galls should be removed from plants and burned before they rupture.
Head smut (Sphacelotheca) reiliana): Can seriously affect yields in dry, hot regions. This is a systemic fungus that enters seedlings without showing symptoms until the tasseling stage. Tassels and ears become deformed and develop masses of black, powdery spores. Head smut is mainly a soil-borne disease. Controls: Most varieties are resistant. Crop rotation and general sanitation also provide some control. Soil applied fungicides in the seed row give fair to good control, but are usually not economical. Seed treatment with a fungicide is ineffective.
Fungal Stalk Rots
Five of the more common fungal stalk rots are discussed below. They attack plants between tasseling and maturity, although Pythium stalk rot may also infect younger plants. Diplodia stalk rot: Most likely to occur several weeks after pollination. The leaves suddenly wilt and die, turning a dull grayish-green, and the stalk dies 7-10 days later. Numerous small,raised,black dots can be seen on the lower internodes of the stalk. Infected portions break readily and can be easily crushed. Diplodia infected stalks usually break between the joints (nodes). Controls: Using resistant varieties, avoiding high rates of N fertilizer without adequate K, and lower plant populations. Gibberella stalk rot: Similar to Diplodia except that the stalks tend to break at the joints, and the inside of the stalk is pinkish-red. The small black dots found on the lower portion of the stalk can be scraped off with a fingernail, unlike those of Diplodia, Controls: See Diplodia, Fusarium stalk rot: Similar to Gibberella and difficult to distinguish from it. Controls: See Diplodia.
Pythium stalk rot: Most likely to occur during long periods of hot, humid weather. Usually attacks a single internode near the soil surface and causes a brown, soft, water-soaked rot that collapses the stem. Stems do not break off but fall over,and plants may remain green for several weeks afterwards. Pythium usually occurs around tasseling time but may also affect younger plants. It is easily confused with Erwinia bacterial stalk rot. Controls: Using resistant varieties.
Charcoal rot (Macrophomina phaseoli) Attacks maize, sorghum, soybeans, beans, cotton, and others, It is most prevalent under very hot, dry conditions and first attacks seedling roots where it produces brown, water-soaked lesions which eventually turn black. The fungus usually does not invade the stalk until well after pollination when it causes the lower internodes to ripen prematurely and shred, causing breakage at the base of the plant, The inner stalk has a charred appearance due to the presence of numerous black dots (sclerotia). Controls: Charcoal rot can be reduced in irrigated fields by maintaining a good soil moisture content during dry spells after tasseling; see also Diplodia.
Fungal Ear and Kernel Rots
Maize can be attacked by a number of ear and kernel rots, especially when very wet weather occurs from silking to harvest. Insect and bird damage of stalks and ears also increases susceptibility.
Diplodia ear rot: Causes early-infected ears to have bleached husks, while normal husks are still green. Ears are shrunken, and the husks seem to be glued together due to the fungus growing in-between. Ears infected later in the season seem normal from the outside but have a white mold that usually starts at the base of the kernels. In severe cases, black fruiting bodies can be seen on the husks and on the sides of the kernels.
Controls: Ears that mature with the tips pointed downward are less susceptible. Good husk covering is also helpful as is an early harvest and proper storage at a safe moisture content. Gibberella ear rot (G. zeae): More prevalent in cool, humid areas and causes a pink to bright red rot starting at the ear tips. G. fujikuroi is the most common ear rot worldwide and is similar in appearance. Both types also produce a cotton-like pink growth over the kernels, and infected grain is toxic to humans, pigs, and birds. Controls: See Diplodia. Fusarium ear rot: Favored by dry, warm weather end similar to Gibberella. Nigrospora ear rot: Causes the cob to be discolored and easily shredded. The interior is gray instead of white. Kernels are poorly filled and can be easily pushed into the partially rotted cob. Spore masses in the form of black spots are found at the base of the kernels. Controls: Balanced soil fertility; see Diplodia.
Maize Bacterial Diseases
Erwinia stalk rot: Causes symptoms similar to Pythium (see fungal stalk rots). Controls: Using resistant varieties and good drainage. Bacterial leaf blight (Stewart's wilt): Transmitted by certain types of maize beetles and by the seed. Sweet maize is more susceptible. Symptoms are pale green to yellow streaks on the leaves, usually appearing after tasseling. The streaks die and may kill the leaf. The stem may also become infected, leading to wilting of the plant. Controls: Using resistant varieties, early use of insecticides to control insect vectors.
Maize Viral Diseases
Maize is attacked by some 25 virus or virus-like diseases which are transmitted mainly by aphids and leafhoppers. Alternate host plants like Johnsongrass, sorghum, and sugarcane play an important role in the spread of most of them, Symptoms can be confusing and may often be caused by other problems such as nutrient deficiencies. Some of the more prevalent viruses are dealt with below:
Maize streak virus: A major problem in many areas of Africa and transmitted by several species of leafhopper (Cicadullina spp.). Early signs are tiny round scattered spots on the youngest leaves which enlarge parallel to the leaf veins. Broken yellow streaks later appear and run along the veins. Controls: Resistant varieties; leafhopper control. Maize dwarf mosaic: Spread by several types of aphids and a wide range of alternate hosts, including Johnson-grass (a sorghum relative) and sorghum. Leaves of infected plants develop a yellowgreen mosaic pattern, mainly on the bases of the younger leaves. Foliage becomes purple or purple-red as plants mature, severe stunting may occur, and few plants produce normal ears. Controls: Using resistant varieties. Destruction of alternate hosts and insect control. Maize stunt virus: Spread by several types of leafhopper (Dalbulus, Baldulus, Graminella) and known as "achaparramiento" in Latin America. Now thought to be a viruslike organism. The Mesa Central strain causes yellowing of the young leaves which later turn red. The Rio Grand e strain produces spots at the bases of young leaves, followed by a yellow striping. Controls: Resistant varieties; insect control. Sugarcane mosaic: Occurs where maize is grown next to sugarcane and causes yellow spots and streaks. Controls: Using resistant varieties of sugarcane.
Sorghum
Fungal Diseases
Seed rots and seedling blights: See maize.
Downy mildews: Sorghum is attacked by three species of downy mildew (S. macrospora, S sorghi, S. graminicola). (Refer to maize for details). Controls: Using resistant varieties and rotation with broad-leaf crops. Many forage-type sorghums are very susceptible to sorghum downy mildew (S. sorghi) and should not be planted on ground where grain sorghum will be sown if the disease is present. Covered kernel smut (Sphacelotheca sorghi): Carried by the seed and penetrates the young seedlings. Plants appear normal until heading time when the kernels are replaced by light-gray or brown, cone-shaped smut galls full of black spores. Controls: Seed treatment with a fungicide is very effective since the spores are carried on the surface. Resistant varieties have been developed.
Loose kernel smut (S cruenta):
Very common in Asia and Africa. As with covered smut, the spores are carried on the planting seed and invade young seedlings. Long, pointed smut galls are formed on the grain heads, and infected plants may be stunted and show increased tillering Unlike covered smut, loose smut spores may cause infections of late emerging grain heads on otherwise healthy plants. Controls: Same as for covered smut. Head smut (S. reiliana): The most damaging of the smuts. Destroys the entire head and replaces it with a mass of dark brown, powdery spores A large gall covered with a whitish membrane bulges out of the boot at heading time. The gall ruptures and spores are scattered by wind and rain over the soil where they survive to infect the next crop. Controls: Seed treatment will prevent the spread from field to field, but will not stop infection from spores already in the field. Resistant varieties should be used and infected plants removed and burned.
Grain (Head) Molds
These are caused by several species of fungi that are most prevalent when sorghum matures during wet weather. Seed becomes heavily molded and will germinate poorly if planted. Controls: Photo-sensitive varieties escape head mold by maturing during drier weather. Other types can be sown to mature during drier weather. Open-headed varieties are somewhat less susceptible than those with compact heads. Work in India has shown that head molds can be reduced by spraying the heads with Captan or Benlate (benomyl) plus a sticker immediately after a heavy rain, but this may not be cost effective. Sorghum Rust
This is caused by the fungus Puccinia purpurea which produces raised brownish pustules on both sides of the leaves. This disease is most common under high humidity but is usually confined to the older, mature leaves. Controls: Using resistant varieties. Fungicides are not usually economical.
Anthracnose
This disease is caused by the fungus Collectotrichum graminicola which attacks the leaves, producing tan to reddish lesions that are round to oval and have soft, sunken centers. It may also cause a stalk rot called red rot. Controls: Using resistant varieties. Other Fungal Leaf Spots
Sooty stripe (Ramulispora sorghi), zonate leaf spot (Gloesocercospora sorghi), and oval leaf spot (Ramulispora sorghicola) are the main fungal leaf spots in West Africa, along with anthracnose. Controls: Resistant varieties offer the best means of control. Removal of host plants like Guinea-grass, Bermudagrass, and Paragrass helps.
Fungal Stalk Rots
Charcoal rot (Macrophomina phaseoli see maize): A serious disease of dryland sorghum. Losses are increasing in India, Ethiopia, Tanzania, and Upper Volta. It is the most serious sorghum disease in Nicaragua and also causes serious losses in Mexico and Colombia. Charcoal rot is especially severe when grain filling takes place during high soil temperatures and drought. Controls: See maize. Milo disease (Periconia circinata): Presently confined to the U.S. and attacks the roots as well as the stalks. Even young plants may be affected. The first symptoms are stunting and slight leaf rolling. The tips and margins of older leaves turn light yellow, and all the leaves eventually become affected. Splitting the base of the stalk lengthwise reveals a dark red discoloration in the center. Roots are also dark red. Controls: Resistant varieties. Red stalk rot (Collectotrichum graminicola): The stalk rot phase of anthracnose. The outside basal portion of the stalk becomes red or purple. If the stalk is split lengthwise, the inner pith shows a reddish discoloration which may be continuous or blotchy. The flower stem may be similarly affected. Controls: See anthracnose.
Bacterial Diseases
Several bacterial leaf diseases attack sorghum and are favored by warm, humid weather. Yield losses usually are not serious. Seed treatment with a fungicide, crop rotation, and resistant varieties are the best controls. Sorghum Viral Diseases Maize dwarf mosaic and sugarcane mosaic produce very similar symptoms on sorghum. The mottled light and dark green mosaic pattern is usually most prevalent on the upper two to three leaves and often includes longitudinal white or yellow streaks. Varieties with a red pigment may show a "red leaf" symptom consisting of red stripes with dead centers. Controls: see maize. Yellow sorghum stunt: A virus-like organism that is spread by leafhoppers. Plants become dwarfed with leaves bunched together at the top. Leaves develop a yellow cream color. Controls: Resistant varieties; insect control.
Millet
Downy mildew (Sclerospora graminicola): Can attack millet as early as the seedling stage. The systemic fungus causes the leaves to become yellowish and under wet conditions a downy white mildew may occur on the undersides of the leaves. Affected seedlings may die within a month without producing any tillers. The symptoms may first appear on the upper leaves of the main stem or on the tillers. The first leaf affected normally shows damage only on the lower portion, but subsequent leaves suffer increasing infection. Heads may be partially or totally deformed. Control: Many local varieties have good resistance. The International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) achieved excellent control of downy mildew by treating planting seed with a newly developed systemic fungicide from Ciba Geigy known as GCA 48/988.
Grain smut (Tolypossporium penicilliriae): Fungi infect the young millet florets on the seed head and replace them with plumb galls ful of black powdery spores. Controls: Use resistant varieties and general sanitation. Seed treatment with a fungicide is probably not very effective. Ergot (Claviceps fusiformis): Common,but generally not serious. The airborne fungal spores infect the young florets before grain development and produce a sweet sticky liquid called honeydew, which is pink or red. The grain head later takes on a bottle-brush appearance due to the formation of dark-colored hard structures called sclerotia Controls:
Burn infected heads. Rust (Puccinia penniseti): Sometimes serious on late millet but usually not with early millet. Leaf spots: Several fungal leaf spots attack millet but are usually not serious.
Peanuts
Foliar Fungal Diseases
Foliar fungal diseases can seriously reduce yields of both nuts and hay, and the decaying fallen leaves provide organic matter for incubating soil-borne diseases like Southern stem rot. Cercospora Leafspot: Attacks peanuts worldwide, but Virginia types (see Chapter 3) are somewhat less susceptible than the Spanish-Valencia types. It is encouraged by wet conditions. Symptoms: Two species of Cercospora fungi are involved. Early leafspot (C. arachidicola) is usually the first to appear and produces round, brownish-red spots surrounded by a yellow halo. Late leafspot (C. personata) occurs later in the season and produces darker spots that may or may not have halos. Both leafspots may also occur on the stems and leaf petioles (leaf stems) as the disease progresses. Severe defoliation can result, which affects yields as well as the performance of mechanical pullers, which require bulky bushes for satisfactory operation. Controls: Crop rotation helps reduce early infections. Even though Virginia types show some resistance, foliar fungicides are usually essential in most cases and are applied as preventatives. Peanuts are a relatively high value crop, which makes use of foliar fungicides very economical. Specific recommendations are given in the next unit. Peanut Rust (Puccinia arachis): This disease is presently confined to Latin America and the Caribbean. It causes small orange to brown raised pustules on the leaves, mainly on the undersides. It can spread rapidly under hot, humid conditions, and leaf drop can be severe. The stems, petioles and pegs can also be affected. Controls: As with leafspot, fungicide sprays or dusts are the only effective control.
Ground Diseases
Ground diseases caused by fungi are sometimes hard to detect and identify and can drastically reduce yields. Southern Stem Rot: Also known as Southern Blight, wilt and white mold, it is the most serious and widespread ground disease attacking peanuts and also affects beans, soybeans, other legumes, potatoes, tomatoes, and other crops. It is favored by warm, wet conditions. Symptoms: In the early stages, some of the leaves on a few branches usually turn yellowish. Under wet conditions, a white cotton-like mold occurs on the lower stem near the soil surface and on any decaying organic debris on the soil. Fungal bodies called sclerotia appear on the affected areas and are light brown to brownish red and about the size of mustard seeds. The leaves begin a gradual wilt, but at first seem to recover at night. Eventually, the entire plant can die. The pegs are destroyed, leaving many pods imbedded in the ground. The disease can also cause pod rot. Controls: There is no way to control this disease once plants are affected, but it can be effectively suppressed through a combination of chemical and cultural controls given below:
- Crop rotations with maize, sorghum, and other grass family plants.
- Deep burial of all crop residues using a moldboard plow. Coarse trash like maize and sorghum stalks need to be chopped up manually or with a disk harrow before plowing. Residues left on the surface serve as a breeding ground for the fungus.
- Planting peanuts on a flat field or on a ridge. Seed furrows should not have depressions which cause poor drainage.
- Avoiding cultivation which throws soil into the crop row, especially when plants are young. This can cause stem injury and burial of young plants, which greatly increases susceptibility to stem rot and crown rot.
- Control of Cercospora leafspot and other foliar diseases with fungicides to minimize defoliation, since fallen leaves also serve as breeding grounds for the fungus.
- Applications of soil fungicides like PCNB (Terrachlor) and Vitavax (Carboxin) in a band over the row at planting or at early pegging stage. These give fair to good protection where stem rot problems are serious. (See the next unit for specific recommendations.)
Seed Rot and Seedling Blight (Preand Post-Emergence "Damping Off")
Pre-emergence rot: It is not unusual to find germinating peanut seeds rotting in the ground. Affected seeds break down rapidly, but early examination will show them to be covered with a growth caused by various species of fungus. Seedling blight is often referred to as Aspergillus crown rot and is caused by Aspergillus niger, a black sooty fungus. True crown rot is more accurately used to describe the disease when it attacks older plants past the seedling stage. The stem tissue just below ground level is attacked on young seedlings shortly after they emerge, and the fungus quickly spreads up the stem, covering it with a mass of black spores. The stem will then suffer a total collapse. Contributing factors: Soils that have been continually cropped to peanuts for long periods have more problems with seed rots and seedling blights. Excessively deep planting weakens the stem and increases susceptibility. Seeds may also be damaged as they are being deshelled. Controls: Seed treatment with fungicides gives good control; usually a combination of two fungicides is needed to provide control of all species. Recommendations are given in the next unit. Attention should also be given to planting depth and crop rotation.
Sclerotinia Blight
This is somewhat similar but less common. Affected plants have a white fungal growth attached to rotted areas of the stem which may extend from below the soil surface up into some or all of the individual runners. Infected stem tissue is very shredded and contains many black fungal bodies. Pegs and nuts are also attacked. Control is usually not needed,but a fungicide called Botran (dicloran) is sometimes applied as a spray in the U.S.
Peg and Pod Rots
Several types of fungi including Sclerotium and Sclerotinia attack the pegs and pods. Soil sterilants are sometimes applied before planting in the U.S., but this would seldom be economical or feasible for small farmers. Crop rotation is helpful. Aspergillus flavus is a fungal mold that attacks stored seed but is sometimes found in the field. Under certain conditions, some strains of A. flavus produce aflatoxin, a potent carcinogen (cancer-causing agent) and toxin that can affect birds, humans, and other mammals. Harvested pods are free of aflatoxin except where they have been broken or damaged by termites, hoeing, threshing or rough handling. The development of Aspergillus and other storage molds largely can be prevented by timely harvest, separation of damaged kernels, and rapid drying of moist pods. Viral Diseases Rosette virus: The most serious disease of peanuts in Africa, especially in the wetter areas. It is spread by one species of aphid (Aphis craccivora) and has several alternate host plants, including Euphorbia hirta, a weed. Plants become severely stunted, and the younger leaves turn yellow and mottled. Emerging leaves remain small and become curled and yellow. Early planting and close spacing appear to reduce the incidence of rosette virus. Affected plants should be removed and destroyed, and aphid control should be considered.
Destruction of alternate host plants is helpful. Resistant varieties have been developed in Senegal. Spotted wilt virus: Caused by tomato wilt virus and spread by several types of thrips. Affected plants have leaves with light green and yellow patterns, often in large patches or in the form of ring spots. Leaves are usually misshapen and puckered, and the plants take on a bunched appearance. Tomatoes, potatoes, lettuce, peppers, ornamental plants, and several types of weed serve as alternate hosts. It is usually not serious.
Beans
Seed-Borne Diseases
Beans suffer heavy disease losses worldwide, and one of the major reasons is the high prevalence of seed-borne diseases. According to CIAT, more than half of the major bean diseases can be transmitted by the seed; these include anthracnose, damping off, root and stem rots, bacterial wilt, bacterial blight, and several viruses. Disease-free certified seed is very difficult to obtain in Latin America and presently makes up less than 3 percent of the bean seed planted there.
Control of seed-borne fungi: Many fungi are carried on or in the seed coat, and seed treatment with conventional fungicides like Arasan (thiram) and Captan (Orthocide) will control them. Others like anthracnose are carried deeper in the seed and are usually unaffected by seed treatment. Systemic fungicides like Benlate (benomyl) have shown some promise in these cases. Foliar applications of systemic fungicides during the latter half of the growing season have significantly reduced the incidence of seed-borne anthracnose in the harvested seed, but are expensive. Delayed harvesting and pod contact with the soil surface during growth can increase seed-borne disease problems.
Control of seed-borne bacteria: Seed treatments will not control internally-borne bacterial diseases on beans. Seed produced in drier areas using strict sanitary and cultural practices such as crop rotation and inspection is less likely to be contaminated.
Control of seed-borne viruses: Current seed treatments are ineffective against seed-borne viruses. Control involves the production of disease-free seed in areas where vectors and hosts can be controlled.
Fungal Diseases
PreEmergence Rot: Seed treatment with fungicides is very effective. (See maize and peanuts.) Root Rots: Beans are very susceptible to root rots caused by Rhizoctonia, Fusarium, Sclerotium, and other fungi. Symptoms include reddish or brown lesions on the hypocotyls (belowground portion of the stem) and rotting of the lateral roots from one to several weeks after emergence. Wilting and leaf yellowing may or may not occur.
Controls:
- In temperate areas, planting only after soils have warmed up
- Good drainage
- Crop rotation
- Avoiding contamination of virgin ground with unclean tools, animal or green manure containing bean residue or dirty irrigation water.
- Treating seed with Arasan (thiram), Zineb, Demosan, PCNB, Vitavax (carboxin) or Benlate at 1-3 active ingredient per kg to give partial control.
- Applying Benlate or PCNB over the seed furrow after planting to give good control.
Anthracnose (Colleotrichum lindemuthianum): Anthracnose is of worldwide importance in cool to moderate temperatures and wet conditions and is spread by seed, soil, crop debris, rain, and tools. It produces elongated reddish-brown to purple cankers on stems and leaf veins. Pods have sunken spots with pink centers and darker borders. Infected seeds mav be discolored and have dark brown to black cankers. Anthracnose is seldom a problem in hot, dry areas.
Controls:
- Use disease-free seed.
- Do not grow beans more than once every two or three years on the same field (includes cowpeas, lima beans).
- Avoid working in fields when the plants are wet.
- Plow under bean residues.
Seed treatment with fungicides is only partially effective. Preventative applications of foliar fungicides have variable results. Rust (Uromyces phaseoli): Rust is of worldwide distribution and also attacks cowpeas and lima beans. Losses are heaviest when plants are infected at or before flowering. The disease is favored by damp weather and cool nights and can infect both the leaves and the pods. First symptoms usually appear on the lower leaf surface as whitish, slightly raised spots. The spots grow into reddish-brown pustules which may reach 1-2 mm in diameter within a week. The entire leaf begins to yellow, then turns brown and dies. Rust is not carried on the seed, but the spores persist in bean residues. There are many races of rust, and bean varieties vary in their resistance to them.
Controls:
- Crop rotation.
- Sulfur dust or fungicide sprays (see next section).
Angular Leafspot (Isariopsis griseola): This disease causes gray or brown angular lesions on the leaves which eventually lead to premature defoliation. Pods may be affected with oval to round spots with reddish-brown centers and seeds may be shrivelled. The disease is carried by the seed, but contaminated plant debris is a much more common source of infection. Control: Using disease-free seed, crop rotation, and removing previously infected crop debris from the field before planting. Seed treatment with a fungicide (Benlate has given good results) and fungicide sprays may help. Sclerotinia Blight (white mold): Causes water-soaked lesions and a white mold on leaves and pods (see also peanuts). It can be controlled by crop rotation and foliar sprays of Benlate, Dichlone, Dicloran, PCNB or Thiabendazole around early to mid-bloom. Irrigation intensifies this disease.
Web Blight (Thanatephorus cucumeris) This disease can be a major limiting factor to bean production under high temperature and humidity. Many other crops are also affected. The fungus causes small round water-soaked spots on the leaves which are much lighter than the surrounding healthy tissue and look like they have been scalded. Young pods show light tan spots that are irregular in shape but become darker and sunken with age--they can be confused with anthracnose. The stems, pods and leaves become covered with a spider web-type growth that is imbedded with brown fungal bodies. Web blight can be carried by the seed but is more commonly transmitted by wind, rain, tools, and the movement of humans and draft animals through the field.
Controls:
- Disease-free seed.
- Crop rotation with maize, grasses, tobacco, and other non-hosts.
- Planting beans in rows, not by broadcasting, to maximize air circulation.
- Fungicide sprays give fair to good control. Systemics like Benlate are recommended under high rainfall.
Bacterial Diseases
Common Blight (Xanthomonas phaseoli) and Fuscous Blight (Xanthomonas phaseoli var. fuscans): Both diseases produce the same symptoms on leaves, stems, pods and seeds. The first leaf symptoms are water-soaked spots on the undersides which grow irregularly and are surrounded by a narrow zone of lemon yellow tissue. These spots eventually become brown and dead. The stem may become girdled near the soil and break. Water-soaked spots form on the pods, gradually enlarge and become dark, red and somewhat sunken. Infected seed may rot and shrivel.
Controls:
- Disease-free seed.
- Crop rotation and deep plowing.
- Copper-base fungicides have controlled leaf symptoms well, but have not given good yield increases. Antibiotics should not be used due to the danger of causing mutations.
- Seed treatment is not very effective.
- Some varietal resistance is available.
Halo Blight (Pseudomonas phaseoli-cola): This bacterial disease prefers cooler temperatures than common and fuscous blights. The initial symptoms are small,water-soaked spots on the undersides of the leaves, which eventually become infected with greasy spots if the attack is severe. Stem girdle or joint rot occurs at the nodes above the seed leaves when the disease results from contaminated seed. However, leaf yellowing and malformation may occur without many other external signs.
Controls:
- Deep plowing and crop rotation
- Removing infected plant debris from the field
- Avoiding work in the fields when the foliage is wet
- Disease-free seed
- Varieties that have some resistance
- Seed treatment with Streptomycin (2.5 g active ingredient per kilogram of seed) or Kasugamycin (0.25 g active ingredient per kilogram), using the slurry (liquid) method.
- Copper-base fungicides applied to the leaves gives poor to fair control.
Viral Diseases
Beans are attacked by a number of viruses, many of which also attack cowpeas. Bean common mosaic, bean yellow mosaic and cucumber mosaic viruses are spread by aphids. Bean rugose mosaic and several others are spread by beetles. Bean golden mosaic and chlorotic mottle viruses are spread by white-flies, and curly top virus by the beet leafhopper. Symptoms include one or more of the following: green-yellow leaf mottling, leaf malformation, puckering, curling, plant stunting, and yellowing. Control consists largely of using resistant varieties and disease-free seed, and controlling insects.
Non-Parasitic Diseases
Seed injury: Bean seed is very susceptible to seedcoat damage and internal injury by improper threshing and mechanical harvesting or by rough handling. Damage may be invisible or produce cracks in the seedcoat, both of which can cause the following seed abnormalities:
- Reduced germination and seedling vigor: This can also be caused by bacteria, fungi, insects, fertilizer burn, and herbicide injury.
- "Baldhead": The seedling lacks a growing point. There is only a bare stump above the cotyledons, so no further leaf growth can occur.
- Detached cotyledons: Young bean seedlings need at least one complete cotyledon or two broken ones with more than half attached to provide adequate nutrition for emergence and early growth.
Dry bean seed (14 percent moisture or below) is the most easily damaged. Bagged seed should not be dropped or thrown onto hard surfaces.
Sunscald: Intense sunlight, especially following cloudy and humid weather, can produce small water-soaked spots on the exposed sides of leaves, stems, branches and pods. These spots turn reddish or brown and may grow together into large necrotic lesions. Air pollutants and tropical spider mites can produce similar symptoms.
Heat Injury: High daytime temperatures may cause lesions that form a constriction around the stem at the soil line, especially on light-colored sandy soils. Temperatures above 35.5°C cause blossom drop if they occur during flowering.
Chemical disease control recommendations
Seed Treatment With a Fungicide
Effectiveness
- Seed rots (pre-emergence damping off): Very good.
- Seedling blights (maize, sorghum, millet, peanuts): Fair.
- Seedling root rots: Poor to fair.
- Seed-borne fungal diseases: Very good if the spores are carried on or close to the seedcoat surface as with loose smut and covered smut of sorghum. Poor if the disease is deeper inside the seed as with bean anthracnose.
- Seed-borne bacterial diseases: Poor.
- Seed-borne viruses: Ineffective.
Seed treatments are very economical and are recommended for all the reference crops, especially for peanuts and the other pulses. They are most beneficial under wet conditions, particularly in cool weather where germination is slowed.
How to Treat Seed
Seed from commercial or government sources may come pretreated with a fungicide or fungicide/insecticide combination. Check the label and look for a red, purple or green dust on the seed. Farmers can treat seed by mixing it with the correct amount of fungicide dust. Large quantities of seed can easily be treated using an oil drum set up to rotate on its longitudinal axis in an offset manner, but bean and peanut seed must be treated gently. Some treatments are applied as slurries (liquids); farmers should always follow label instructions.
A mixing drum for applying insecticide and/or fungicides to seeds prior to planting.
Precautions: With the exception of mercury compounds like Ceresan, Semesan, and Agallol, seed treatment fungicides are relatively non-toxic, although some can cause skin and eye irritation. Avoid using mercury compounds. NEVER use any treated seed for human or animal consumption. Combination fungicide/ insecticide treatments containing Dieldrin or other Class 1 and 2 compounds should be handled with rubber gloves.
Table 10 Recommendations for Seed Treatment
The following recommendations are based on current information from North Carolina State University and CIAT
|
TREATMENT |
Grams/kg |
Oz/100 lbs of seed | |
|
Maize |
Arasan (thiram) 50% dust |
1.5 |
2.5 |
|
& |
Captan (Orthocide) 75% dust |
0.75 |
1.25 |
|
Sorghum |
Dichlone (Phygon) 50% dust |
0.6 |
1.0 |
|
Peanuts |
Arasan (thiram) 50% dust |
2.0-2.5 |
3.0-4.0 |
|
Captan + Maneb (30-30 dust) |
2.0-3.0 |
3.0-5.0 | |
|
Botran + Captan (30-30 dust) |
2.0-3.0 |
3.0-5.0 | |
|
Difolatan + Captan (30-30 dust) |
2.0-3.0 |
3.0-5.0 | |
|
Vitavax (carboxin) 75% W |
2.0-3.0 |
3.0-5.0 | |
|
Vitavax + Arasan or Captan |
1.25-2.0 |
2.0-3.0 | |
|
Beans Arasan, Captan, Zined, Busan or Vitavax |
.0-3.0 |
- |
NOTE: Increase dosage on sorghum by about 25-50%, since it has more surface area per kg.
NOTE: The combinations are recommended where Aspergillus seedling blight is prevalent. Vitavax is a systemic fungicide. Innoculated peanut seed should be treated immediately before planting.
NOTE: Seed coat infections of anthracnose have been effectively controlled with Arasan 75% dust applied at 5g/kg of seed.
Recommendations for Soil Fungicides
Vitavax (carboxin) and PCNB (Terrachlor) are sometimes applied to the seed furrow at planting or to the row soil during crop growth to control soil-borne diseases like Southern stem rot and root rot. They are rarely necessary or economical for maize, sorghum, and millet and are usually not justified on peanuts an-i beans unless potential yields are high and disease problems are serious.
Peanuts
Southern stem rot control: Apply PCNB preplan" at active ingredient 11kg/ ha in a band 20-30 cm wide centered over the row or at early pegging stage in a band 30-40 cm wide. Preplant applications should be incorporated 5.0-7.5 cm deep. When applying PCNB at early pegging, direct the spray so that it reaches the soil at the base of the plants. If granules are used, don't apply them when the plants are wet. Drag bags over the plants to settle the granules to the soil. Vitavax can be applied in the same manner at early pegging using 1.1-2.25 kg/ha active ingredient (Recommendations from North Carolina State University and Clemson University.)
Beans
Root and stem rot caused by Sclerosium rolfsii: Application of PCNB at 3.4-4.4 kg/ha of active ingredient to the seed and surrounding soil at planting has proven effective in Brazil (CIAT data). Root rot caused by Rhizoctonia solani: North Carolina State University recommends PCNB at 100-150 grams active ingredient per 1000 meters of row length applied at planting time to the seed and surrounding furrow soil.
Recommendations for Foliar Fungicides
Protectant versus Erradicant Fungicides
Most fungicides like Maneb, Zineb, Difolatan, and Manzate act as protectants by remaining on the leaf surface to prevent fungal spores from germinating and penetrating the plant. They have little or no erradicant ability to stop the progress of an existing infection. However, a few fungicides like Benlate (benomyl) and Thiabendazole (Mertect) are actually absorbed into the leaf tissue and translocated outwards toward the margins. These systemic fungicides act as erradicants as well as protectants and also have other advantages:
- They are not vulnerable to being washed off the foliage by rainfall or sprinkler irrigation.
- Since they are translocated within the leaf, uniform foliage coverage is not as important as with the nonsystemic protectant fungicides.
The main disadvantage of the systemic fungicides is that they are effective against a narrower range of fungal diseases than most of the protectant fungicides, so more care must be taken to match the product to the disease. Vitavax (carboxin) and Plantvax (oxycarboxin) are two other systemic fungicides mainly used for seed treatments and soil application.
Guidelines for Applying Foliar Fungcides
Type of Crop: Foliar fungicides are seldom economical for maize, sorghum, and millet. They will give the best benefit/cost ratio when used on wellmanaged peanuts and beans under conditions where fungal leaf diseases are a limiting factor. When to Apply: Ideally, applications should start slightly before the onset of infection or at least before the disease signs have become very evident. This is especially important when non-systemic protectant fungicides are used. In most growing areas, the major fungal leaf diseases are somewhat predictable as to their date of first appearance. Fungicides are too expensive to be used on a routine basis from the time the plants emerge. Besides, most fungal diseases do not infect plants until around flowering time or after. Frequency of Application: This depends on disease severity, rainfall, and type of fungicide. The non- systemic protectant fungicides can be washed off the foliage by rainfall (or sprinker irrigation), but the systemics remain safely within the plant once they have been absorbed. Under frequent rainfall, the protectants may have to be applied as often as every four to seven days. Under drier conditions, intervals of 10-14 days are normal. Systemics are usually applied once every 12-14 days regardless of rainfall frequency. Disease severity also affects application frequency but is usually closely related to rainfall and humidity (as well as varietal resistance).
Uniform and thorough coverage of crop foliage is very important when applying fungicides. This is especially true for the protectant products which are effective only on those portions of the leaf surface they actually cover. An attempt should be made to cover both sides of the leaves when using protectants. Stickers and spreaders are recommended for nearly all fungicide sprays to enhance coverage and adhesion.
Duter is one exception, since these additives increase the likelihood of crop injury from that particular product. Some fungicides already contain stickers and spreaders, so be sure to read the label. Amount of water needed for adequate foliage coverage: This varies with plant size, crop density, and type of sprayer. When using backpack sprayers on full-size plants, at least 700 1/ha of water is needed.
Dosage Recommendations
Label instructions and extension service recommendations are the specific quidelines to follow. The following recommendations are meant to serve as general guidelines. Peanut Cercospora Leafspot: Benlate and Duter have generally proved the most effective, although most other products, such as Dithane M-45, Antracol, Bravo (Daconil), Difolatan, copper-sulfur dusts, and copper-base sprays, also give satisfactory control. The following recommendations come from North Carolina State University (U.S.A.) and Australia.
Duter 47% WP, 425 g actual formulation per hectare. Do not use a sticker or a spreader.
Benlate 50% WP, 425 g actual formulation per hectare plus stickerspreader.
Control is enhanced by combining 285 g Benlate plus 1.7 kg Dithane M-45 or Manzate 200 plus 2.3 non-phytotoxic crop oil per hectare. The oil improves penetration.
Daconil (Bravo), 875-1200 g active ingredient per hectare.
Copper-base products like copper oxychloride, copper hydroxide, and basic copper sulfate can be used at 1.85 kg active ingredient per hectare.
Antracol 70% WP can be used at 1.7 kg/ha.
Copper-sulfur dust: Follow manufacturer's recommendation.
Note: Do not feed treated hay to livestock unless only copper or copper-sulfur products are used. Duter helps retard spider mite buildup. Plant injury may result if a sticker-spreader is used with Duter.
Bean Leaf Diseases: Potential bean yields must be high to warrant the use of foliar fungicides. Systemics should be considered where rainfall is high if they are effective against the disease involved.
Anthracnose: Literature from CIAT recommends Maneb 80% W or Zineb 75% W at 3.5 g/l of water, Benlate at 0.55 g/l, Difolatan 80 W at 3.5 kg/ha, and Duter 47 W at 1.2 g/l.
Rust: Suggestions from CIAT are for Dithane M-45 or Mancozeb at 3-4 kg/ha; Manzate D 80 W or Maneb 80 W (Dithane M-22) at 4 kg/ 1000 1/ha; sulfur dust at 25-30 kg/ha. Plantvax (oxycarboxin), a systemic, has been found effective when sprayed at a rate of 1.8-2.5 kg/ha of the 75% WP at 20 days and 40 days after planting or every two weeks until the end of flowering.
White mold (Sclerotinia): North Carolina State University recommends Benlate 50 W at 1.72.25 kg/930 1/ha on Botran (dichloran) 75 W at 4.5 kg/9301/ha.
Web blight: Recommendations from CIAT are for Benlate 50 W at 0.5 kg/ha (0.5 g/l at 1000 1/ ha) or Brestan 60 at 0.8 kg/ha or Maneb (DIthane M-22) at 0.5 g/liter. (Note: The Maneb dosage seems unusually low.)
Angular leafspot: Literature from CIAT suggests Benlate 50 W at 0.5 g/l, Zineb, Mancozeb, Ferbam-sulfur-adherent (no dosages given).
Bacterial blights: Use copperbase sprays and follow label directions.
Nematodes
Nematodes are tiny, colorless, thread-like roundworms 0.2-0.4 mm long. There are many kinds of plantfeeding nematodes. Most live in the soil and feed on or within plant roots using needle-like mouthparts for piercing and sucking. They dissolve the roots' cell contents by injecting an enzyme with produces various reactions depending on the type of nematode. The root-knot nematode causes portions of the roots to swell into galls or knots, while root lesion nematodes produce produce dark-colored lesions on the roots. Sting nematodes and stubby-root nematodes prune the root system and make it appear stubby. Root growth is often stopped and becomes very susceptible to attack from bacteria and fungi.
Nematodes are most prevalent and active where soil temperatures are warm. They seem to prefer sandier soils or those portions of the soil where fertility or moisture are low. However, clayey soils can have serious nematode problems, too.
Since they are so tiny, nematodes seldom move more than a few inches a year. Unfortunately, they are spread easily by soil carried on tools and equipment or by water runoff from a field.
Maize, sorghum and millet are fairly resistant to most kinds of nematodes, and yield losses seldom exceed 10-15 percent. The pulses are most vulnerable to root lesion and sting nematodes which feed on roots, pegs, and pods. Beans and cowpeas are attacked by root knot, root lesion, and sting nematodes plus several other types. In Kenya, heavy infestations of root knot nematodes have reduced bean yields by up to 60 percent in some cases.
Diagnosing Nematode Damage
Above-ground symptoms are seldom distinctive enough to make a conclusive diagnosis without examining the root system, but the following are possible indications of nematode damage:
- Stunting, yellowing, lack of plant vigor. However, these can be caused by many other problems--low fertility, diseases, excessive soil acidity or moisture, for example.
- Wilting, even when moisture seems to be adequate and heat is not excessive. This can also be caused by soil insects, borers, and diseases.
- Damage almost always occurs in scattered patches in the field and is rarely uniform. This is an important characteristic of nematode problems.
Root symptoms, as described below, may be observed if the roots are carefully dug up and examined:
- Galls or knots are a sure sign of root knot nematode damage. These should not be confused with the Rhizobia bacteria nodules attached to the roots of legumes. The galls or knots caused by root knot nematodes are actually swollen portions of root.
- Other types of nematodes cause tiny, dark-colored lesions, stubby roots or loss of feeder roots. This damage should not be confused with that caused by rootworms, white grubs or other insects.
Root knot nematode galls on bean roots. Note how they differ from nodules by actually being part of the root.
Laboratory diagnosis is usually needed to confirm nematode damage, although root knot nematode injury is often readily apparent. Plant pathology labs in most countries can test soil and root samples for nematodes. It will be necessary to take 10 random subsamples within the field right next to the plants using a shovel for testing. Sample the soil by digging down about 2025 cm and discarding the soil from the top 5 cm and from the sides of the shovel. The remaining soil should be placed in a pail, making sure some roots are included. The sub-samples should be mixed together and a half-liter of the soil placed in a plastic bag. The sample should be protected from sunlight or excessive heat, preferably by refrigerating it until mailing time. A lab diagnosis is also valuable for planning a suitable crop rotation program to reduce nematode numbers, since different types vary in their crop preferences.
Nematode Control
Complete eradication is impossible, but chemical and nonchemical controls can reduce populations to tolerable levels. Non-Chemical Controls Crop rotation: This is sometimes difficult or impractical, since most types of nematodes have many crop hosts as shown below:
- Root crop nematodes (Meloidogyne spp.): Beans, cowpeas, cucumber, squash, watermelon, cantaloupes, tomatoes, tobacco, okra, cotton, carrots, lettuce, peas and strawberries are very susceptible, but peanuts can be attacked also. Grass family crops tend to be less vulnerable. Cotton and peanuts can also be included in the same rotation, since they do not share the same root knot species. However, planting cotton immediately prior to peanuts will cause a buildup of peanut soil diseases.
- Root lesion nematodes (Pratylenchus spp.): Beans, cowpeas, peanuts, soybeans, tobacco, okra, pepper, potatoes, sweet potatoes, tomatoes, sugar cane and strawberries are among the most susceptible. Maize is less so, and sorghum and millet have better resistance.
- Sting nematodes (Bolonolaimus spp.): Beans, cowpeas, cotton, soybeans, maize, millet, sorghum, sweet potatoes, tomatoes, squash and pasture grasses are among the hosts. Tobacco and watermelons are resistant.
Some types of tropical legume tree such as Prosapis spp. harbor nematodes. Host country extension services sometimes have a nematode specialist who should be consulted concerning crop rotations and other controls.
Resistant varieties: Varieties differ somewhat in their resistance to nematodes.
Exposure: Plowing up roots of susceptible crops right after harvest will expose them to sunlight and drying, which will kill many of the nematodes.
Flooding: One month of flooding followed by a month of drying and a further month of flooding will greatly reduce nematode problems, but is not often feasible.
Antagonistic plants: Many garden books recommend inter-planting marigolds among susceptible crops to control nematodes. Unfortunately, research has shown that marigold species vary in their effectiveness, which is limited mainly to one type of nematode, the root lesion nematode. Furthermore, marigolds do not kill nematodes, but starve them out. This means that interplanting is not effective, since the nematodes will still have a food source. Marigolds would have to be planted exclusively and then followed by a crop susceptible to root lesion nematodes in order to provide some control.
Two legume green manure or cover crops, Crotalaria spectabilis (showy crotalaria or rattlebox) and Indigo fera hirsute (hairy indigo) can reduce populations of most type of nematodes.
Soil: Good soil fertility and high soil organic matter levels help somewhat.
Chemical Controls
Soil fumigants: Some of these, like methyl bromide, Vapam, Basamid and EDB are often used on vegetables or transplant beds, but are either too expensive or require specialized application equipment. Some are very dangerous.
Non-fumigant nematocides: These include Mocap (ethoprop), Furadan and Dasanit, and can be applied as granules to the crop row and are effective against some insects. Under small farmer conditions, their use on maize and other cereals for nematodes only would be uneconomical except in cases of heavy infestations and high potential yields. There may be some cases where their use is justified on the pulses, especially peanuts. Product use guidelines for some of the more common nematocides:
NEMAGON (DBCP, Frumazone): Comes as a liquid or granules but has been virtually banned in the U.S. as a possible carcinogen. Prolonged exposure over the years has caused testicular atrophy in males. Stay away from this one.
FURADAN (Carbofuran): See description under Section B. Has a very low dermal, but very high oral toxicity. Nematode application guidelines are:
Peanuts: Apply a band 30-35 cm wide over the row before planting; use 2.2-4.5 kg of active ingredient per hectare. Needs to be worked into the soil 5.5-15 cm deep.
Maize: Apply in a band 18-36 cm wide over the row before planting and work into the top 5-10 cm of soil. Use 1.7-2.25 kg of active ingredient per hectare.
MOCAP (Ethroprop, Prophos): Kills nematodes and soil insects but is very toxic both orally and dermally. Applied like Duradan at the rate of 1.7-2.25 kg active ingredient per hectare. Not recommended for most small farmers. Non-systemic.
TEMIK (Aldicarb): A systemic insecticide/nematocide with extremely high oral and dermal toxicity. Avoid it.
DASANIT (Terracur, fensulfothion): A non-systemic product for soil insects and nematodes. Very high oral and dermal toxicity. Avoid using.
NEMACUR (Phenamiphos, Fenamiphos): A systemic product for nematodes, soil insects, and above-ground sucking insects. Class 2 toxicity. Applied to peanuts like Furadan at 1.7-2.85 hectare. Handle with care. Use Furadan instead if possible due to its much lower dermal toxicity.
Bird and rodent control
Birds
Seed-Eating Birds
In parts of Africa and in other areas, birds like the bush fowl dig up and eat freshly-planted seeds, They often uproot young seedlings of maize and other cereals during the first several weeks of growth as well. Controls: Scarecrows are relatively ineffective, although noise-making devices may offer some control. It is often necessary to frighten away the birds from planted fields during their usual early morning and late afternoon feeding times for the first two or three weeks after planting. Farmers sometimes soak their seeds in highly toxic insecticides like Endrin and Dieldrin and plant them or use them as scattered bait. This is not only dangerous, but can lead to indiscriminate killing of wildlife. Some safer repellents are available such as Mesural 50 percent dust, which is mixed with maize before planting at the rate of 9-10 g/kg to repel blackbirds. Mesurol may injure maize seed under cool, wet conditions. Dusting seeds with Captan fungicide or soaking them in turpentine may provide a fair repellent effect.
Perhaps the most effective control method is continuous string flagging which uses cloth or plastic streamers 5-6 cm wide and 50-60 cm long. The streamers are attached at 1.5 m intervals to strong twine which is strung along heavy stakes at least 1.2 m tall spaced about 15 m apart.
Quelea Birds
The Quelea bird (Black-Faced Dioch) is a sparrow-sized weaver that may be the world's most destructive grain-eating bird. It is confined to the Sahel and savanna regions of Africa in a band running from Senegal to Mauritania to Ethiopia and Somalia and then south through East and South Africa and across into Angola.
The birds congregate in vast nomadic colonies that feed on the seeds of both natural grasses and crops like millet, sorghum, rice, and wheat, mainly in the unripe stage. (Maize is less affected.) Queleas begin breeding a few weeks after the rainy season begins and build their nests in thorn trees or swamp grasses. Studies in Senegal have shown that even small trees can hold up to 500 nests and taller ones up to 5000-6000. Each pair of Queleas can produce two young. Controls: In areas prone to attack, the villagers build high platforms in the fields and maintain noise-making vigils, sometimes for many weeks, while the grain is ripening. Governments frequently undertake mass Quelea extermination campaigns which center around the destruction of nesting and roosting sites with explosives, flamethrowers, etc. South African authorities killed 400 million with aerial sprays in one four-year campaign. However, the birds usually return in undiminished numbers within a year or two, since they are highly nomadic and have extensive breeding grounds estimated to cover two million square miles. At the present time, bird-resistant crop varieties are not very successful against the Quelea, and the same seems to be true of repellents like Avitrol and Morkit.
Other Grain-Eating Birds
Blackbirds (grackles, starlings, etc.), sparrows, cockatoos, parrots, galahs and pigeons also feed on grain crops, though usually in less awesome numbers than Queleas. Bird-resistant varieties of sorghum (see Chapter 3) are fairly effective at repelling them while the grain is ripening, but lose this ability when maturity nears. Repellants like Avitol (aminopyridine) are often used successfully in the U.S. The usual result of using repellents in one field, however, is that the birds move on to attack other fields that are unprotected.
Rodents
The cane rat (Thronomya sp.) can cause considerable losses of cereal crops during the latter stages of growth, especially if lodging has been heavy due to high winds or diseases. Controls:
- Rodents can be discouraged from entering fields by maintaining a 2.0-3.0 m wide cleared swath around the field borders from planting until harvest. Fences made from oil palm fronds or split bamboo are also effective, especially if traps or wire snares are set in the gaps.
- Good weed control in the field is helpful.
- Leaning or fallen plants should propped up and the dry lower leaves stripped off to help deter climbing.
- Many villages carry out organized killing campaigns. The best time for such campaigns is during the dry season when the rats congregate in the few remaining pockets of green vegetation.
- Repellants like Nocotox 20 may be partly effective.
- Rats should be prevented from gaining access to stored grains and other food that can cause a buildup in populations during the dry season. (See Chapter 7.)
- Poison baits can be used.
NOTE: Killing rats in the field with poisons, traps, and other methods is usually not a very effective longterm solution. The best approach is to prevent a rat population buildup; this requires area-wide coordination. The PC/ICE Small Farm Grain Storage manual contains a very useful section on rat control.