Fertilizers
Fertilizer use is often the management factor producing the largest increases in reference crop yields. However, yield response is heavily influenced by two factors:
- The control of other limiting factors: Fertilizer usually gives a much better response when used as part of a "package" of improved practices designed to control other major yield limiting factors in addition to soil fertility.
- How fertilizer is used: Good results from fertilizer cannot be expected unless the farmer knows what kind and how much to use, and how and when to apply it.
Aside from water, sunlight, and air, plants need 14 mineral nutrients which are usually grouped as follows:
MACRONUTRIENTS
Primary Secondary
|
NITROGEN (N) |
CALCIUM (Ca) |
|
PHOSPHORUS (P) |
MAGNESIUM (Mg) |
|
POTASSIUM (K) |
SULFUR (S) |
|
MIRONUTRIENTS |
(not primary or secondary) |
|
IRON (Fe) |
ZINC (Zn) |
|
MANGANESE (Mn) |
BORON (B) |
|
COPPER (Cu) |
MOLYBDENUM (Mo) |
The macronutrients make up about 99 percent of a plant's diet. Nitrogen, phosphorus and potassium account for about 60 percent and are definitely the "Big Three" of soil fertility, both in terms of the quantity needed and the likelihood of deficiency (see Table 4).
This does not mean that the secondary macronutrients or the micronutrients are any less essential. True, their deficiencies are not as common, but they can have just as serious an effect on crop yields.
Table 4. Amount of Nutrients Taken Up by a 6300 kg Yield of Shelled Maize
|
Kg |
Micronutrients |
Grams | |
|
Nitrogen |
157 |
Iron |
4200 |
|
Phosphorus (P2O5) |
60 |
Manganese |
1000 |
|
Potassium (K2O) |
124 |
Zinc |
30 |
|
Calcium |
29 |
Copper |
7 |
|
Magnesium |
25 |
Boron |
7 |
|
Sulfur |
17 |
Molybdenum |
|
Nitrogen (N)
Nitrogen is the most commonly deficient nutrient for non-legumes. It promotes vegetative growth and is an essential constituent of protein and chlorophyll (needed for photosynthesis).
Crops vary in their need for N. Crops with a lot of vegetative (leafy) growth have relatively high N needs. These include maize, sorghum, millet, rice, sugarcane, pasture grasses, and most leafy and fruit-type vegetables. Root crops like potatoes, sweet potatoes, cassava (manioc, yuca), and tropical yams have lower N needs, and excessive amounts tend to favor leafy growth over tuber growth (with the exception of most improved potato varieties which have high N needs).
Legumes are able to satisfy part or all of their N needs themselves through the process of nitrofixation. Peanuts, cowpeas, mung beans, pigeonpeas, and chickpeas are usually able to meet their own N requirements in this way. Common beans (kidney beans) are less efficient at N fixation and may need up to half as much N fertilizer as maize. Too much nitrogen can adversely affect crop growth, especially if other nutrients are deficient:
- It may delay maturity.
- It may lower disease resistance.
- It may increase lodging problems in cereal crops.
Available versus Unavailable N
Only nitrogen in the form of ammonium (NH+4) and nitrate (NO-3) in the soil is available to plants. However, about 98-99 percent of a soil's total N is in the unavailable organic form as part of humus. Soil microbes gradually convert this unavailable organic N into ammonium and then nitrate. Most soils are too low in humus to supply N at a rapid enough rate for good yields. That is why N fertilizer is usually needed for non-legumes.
Available soil N can become tied up and unavailable when crop residues low in N are plowed into the soil. This is because the soil microbes that decompose the residues need N to make body protein.
Most crop residues like maize, millet, and sorghum stalks supply large amounts of carbon, which the microbes use for energy, but not enough N for the microbes' protein needs. The microbes make up for this shortage by taking ammonium and nitrate N from the soil. A crop may suffer a temporary N deficiency if planted under these conditions, until the microbes finish decomposing the residues and finally release the tiedup N as they die off. (Occasionally even young legumes will be affected.) This type of N deficiency can be prevented easily by applying about 2530 kg/ha of N at planting time when growing a non-legume. Available N is Easily Lost
Nitrate N (NO-3) is much more readily leached (carried downward away from the root zone by rainfall or irrigation) than ammonium N (NH+4), since it is not attracted to and held by the negatively charged clay and humus particles. (These act like magnets and hold onto positively charged nutrients like NH4+, K+, and Ca++ and keep them from leaching).
The problem is that tropical and sub-tropical temperatures are always high enough to promote a rapid conversion of ammonium N to nitrate N by soil microbes. Most ammonium type fertilizers will be completely changed to leachable nitrate in a week in warm soils. The higher the rainfall and the sandier the soil, the higher the leaching losses of N. The best way to avoid excessive leaching is to apply only part of the fertilizer at planting and the rest later on in the crop's growth when uptake is higher.
Phosphorus (P)
Phosphorus promotes root growth, flowering, fruiting and seed formation. Remember these four vital facts about phosphorus:
- Phosphorus deficiencies are widespread: Much of a soil's native P content is tied up and unavailable. Worse yet, only about 5-20 percent of the fertilizer P applied will be available to the crop since much of it also gets tied up as insoluble compounds. This P fixation is especially a problem on highly-weathered, red tropical soils low in pH (high in acidity).
- Phosphorus is virtually immobile in the soil: Phosphorus does not leach except in very sandy soils. Many farmers apply P fertilizer too shallowly and very little gets to the roots.
- Young seedlings need a high concentration _of P in their tissues to promote good root growth. This means that P needs to be applied at planting time. One study showed that maize seedlings take up to 22 times as much P per unit of length as do 11weekold plants.
- Application method is vitally important and determines how much of the added P gets tied up. Broadcast applications (uniform applications of fertilizer over the entire field) maximize P tie-up and should seldom be recommended for small farmers. Placement in a band, half-circle or hole near the seed is two to four times as effective as broadcasting, especially for low to medium rates of application.
Potassium (K)
Potassium promotes starch and sugar formation, root growth, disease resistance, stalk strength, and general plant vigor. Starch and sugar crops like sugarcane, bananas, potatoes, cassava, and sweet potatoes have particularly high K needs. Maize, sorghum, millet, rice, and other grasses are more efficient K extractors than most broadleaf crops. Remember these facts about K:
- Potassium deficiencies are not as common as those of N and P: Most volcanic soils tend to have good available supplies. However, only the soils lab can tell for sure. Potassium: Only about one or two percent of the soil's total K is in the available form, but this is often enough to satisfy the needs of some crops. The good news is that tie-up of fertilizer K is not usually serious and never approaches that of P.
- Leaching losses are usually minor: The available form of K has a positive (plus) charge. The negatively charged clay and bumus particles act like magnets and attract the plus-charged K to their surfaces to help reduce leaching. However, leaching losses can be a problem on sandy soils or under very high rainfall.
- High K applications can encourage magnesium deficiencies.
The Secondary Macronutrients: Calcium (Ca), Magnesium (Mg), And Sulfur (*)
For most crops, calcium is more important for its role as a liming material (to raise soil pH and lessen acidity) than as a nutrient. Even very acid soils usually have enough calcium to meet the plants' nutrient needs, while soil pH may be too low for good growth. It takes large amounts of calcium to raise the pH compared to those needed for plant nutrition.
Peanuts, however, are an exception and have unusually high calcium needs which usually must be met by supplying gypsum (calcium sulfate.) This is not a liming material.
Magnesium deficiencies are more common than those of calcium and are most likely to occur in sandy, acid soils (usually below pH 5.5) or in response to large applications of K. Too much calcium relative to magnesium also can bring on Mg deficiencies. Farmers who need to lime their soils are usually advised to use dolomitic limestone (about a 50-50 mix of Ca and Mg carbonates). Both calcium and magnesium are slowly leached from the soil by rainfall.
Sulfur deficiencies are not common, but are most likely to occur under these conditions:
- Many volcanic soils tend to be low in available S. Land near industrial areas usually receives enough S from the air.
- Sandy soils and high rainfall
- Use of low sulfur fertilizers (see Table 17). Low analysis fertilizers (those with a relatively low NPK content) generally contain much more
- than high analysis fertilizers such as 18-46-0, 0-45-0, etc.
The Micronutrients
Micronutrient deficiencies are much less common than those of N,P, or K, but are most likely to occur under these conditions:
- Highly leached, acid, sandy soils.
- Soil pH above 7.0 (except for molybdenum which is more available at lower pH's).
- Intensively cropped soils fertilized with macronutrients only.
- Areas where vegetables, legumes and fruit trees are grown.
- Organic (peat) soils.
Table 5 Susceptibility of the Reference Crops to Micronutrient Deficiencies
|
Crop |
Most Common Micronutrient Deficiency |
Conditions Favoring Deficiency |
|
MAIZE |
Zinc |
Soil pH above 6.8; sandy soils; high P |
|
SORGHUM |
Iron |
Soil pH above 6.8; sandy soils; high P |
|
BEANS |
Manganese, Zinc |
Soil pH above 6.8; sandy soils |
|
Boron |
Acid, sandy soils, pH above 6.8 | |
|
PEANUTS |
Manganese, Boron |
Refer to above |
Micronutrient Toxicities: Iron, manganese and aluminum can become overly soluble and toxic to plants in very acid soils. Boron and molybdenum can cause toxicities if improperly applied.
Determining fertilizer needs[edit | edit source]
The amount of nutrients that different crops must absorb from the soil to produce a given yield is fairly well known. Yet proper fertilization is not a simple matter of adding this amount for several reasons:
- The farmer needs to know what share of the nutrients are already in the soil in available form.
- A plant's ability to recover nutrients, whether from fertilizer or natural soil sources, depends on the type of crop, the particular soil's capacity to tie up different nutrients, weather conditions (sunlight, rainfall, temperature), leaching losses, physical soil factors like drainage and compaction, and insect and disease problems.
Likewise, there is no such thing as "tomato fertilizer" or "maize fertilizer", etc. Soils differ so much in natural fertility that no one fertilizer could possibly be right for all types of soil, even for one kind of crop.
When dealing with the reference crops, farmers cannot afford to waste their scarce capital on fertilizers that might be inappropriate for their soils. They also need reasonable guidelines on how much to apply. There are five basic methods used to determine fertilizer needs:
- Soil testing
- Plant tissue testing
- Fertilizer trials
- Spotting visual "hunger signs"
- Making an educated guess
Soil Testing
Soil testing by a reliable laboratory is the most accurate and convenient method for determining fertilizer rates.
Most labs routinely test for available P and K and measure soil pH and exchange capacity (the soil's negative charge). Most do not test for available N, since results are not very accurate.
Some will be able to test for Ca, Mg, S, and some of the micronutrients (micronutrient and tests vary in reliability).
If the soil is overly acid, the lab will usually be able to tell how much lime the soil will need. Most can test the salinity and alkali hazard of the soil and irrigation water (most common in semi-arid to arid areas).
At the very least, the lab will provide an N-P-K application recommendation for the crop involved The better labs will tailor the recommendation of the farmer's yield goal and management ability, based on the farmer's responses to a questionnaire supplied by the lab.
Portable soil test kits are not as accurate as laboratory testing but can give a fairly good estimate of soil conditions at a test site. The instructions state within what limits the test kit is accurate.
These kits give results that are as accurate as most farmers will need for growing reference crops. However, if a soil testing laboratory is available farmers should be encouraged to send samples in. How to Take a Soil Sample
Improper sampling by the farmer or extension worker is the most common cause of faulty lab results. A 200-400 gram sample may represent up to 15,000 tons of soil. The soil laboratory's instructions should be carefully read before sampling. These are usually printed on the sampling box or on a separate sheet. (See Appendix J for general instructions on how, when, and how often to soil test.)
Plant Tissue Tests
The crop can be tissue-tested while growing in the field for N-P-K levels in the sap. Kits cost about US -, but some of the reagents need replacement every year.
Tissue tests are best used to supplement soil test data, since the results can be tricky to interpret by non-professionals. Sometimes plant sap nutrient levels are not well correlated to those in the soil, because weather extremes, insects, and diseases affect uptake. Deficiencies of one nutrient such as N can stunt plant size and cause P and K to "pile up: in the plant sap, giving falsely high readings. The tests are also geared to higher yield levels than most small farmers can reasonably hope to attain. The crops receiving low to moderate fertilizer rates that provide the best return per dollar may show tissue test results indicating deficiencies.
One advantage of tissue testing is that it may be possible to correct a deficiency while the crop is still growing and thus increase yields.
Total Plant Analysis: Some labs can run a total nutrient analysis of plant leaves with a spectrograph, but it may cost US .
When collecting leaf samples, it is important to pay close attention to the kit's or lab's sampling instructions. Taking leaves from the wrong part of the plant will make results invalid.
Fertilizer TrialsSee Chapter 8 and Appendix B.
Spotting Visual "Hunger Signs"
Severe nutrient deficiencies usually produce characteristic changes in plant appearance, particularly in color. Spotting these "hunger signs" can be useful in determining fertilizer needs, but there are several drawbacks:
- Some hunger signs are easily confused with each other or with insect and disease problems. If more than one nutrient is deficient at once, the hunger signs may be too vague for accurate diagnosis.
- Hidden hunger: Hunger signs will not usually appear until the nutrient deficiency is serious enough to cut yields by 30-60 percent or more. This "hidden hunger" can cause unnecessarily low yields even though the crop may look good throughout the growing season.
- It may be too late to correct deficiencies by the time hunger signs appear. Any N applied much beyond flowering time in the cereal crops will increase grain protein more than yields (such protein increases are slight compared to the amount of N used and the yield that is sacrificed by late application). Phosphorus should ideally be placed 7.5-10 cm deep and this is difficult to do without damaging the roots after the crop is up and growing.
Specific hunger signs for the reference crops may be found in Appendix G.
Making an Educated Guess
If no soil test results are available for a farmer's field, a reasonable estimate of N-P-K needs can be made based on at least four or more of the following criteria:
- Available soil test results from nearby farms with the same soil type and a similar liming and fertilizer history.
- Data from fertilizer trials on the same soil type.
- An extension pamphlet on the crop with fertilizer recommendations for the area soils. (Do not rely on their accuracy unless the recommendations are based on soil tests and/or field trial results.)
- The particular crop's relative nutrient needs (discussed later in this section).
- A thorough examination of the soil for depth, drainage, texture, filth, slope, and other factors that may limit yields or fertilizer response, including soil pH (see page 169 on liming).
- Yield history and past management of the farm regarding fertilizer and liming.
- The farmer's management ability, available capital, and willingness to use complementary practices like improved seed, insect control, etc.
Fertilizer types and how to use them[edit | edit source]
Chemical (inorganic) fertilizers are frequently accused of everything from "poisoning" the soil to producing less tasty and nutritious food. Should the extension worker encourage client farmers to forget about chemical fertilizers and use only organic ones (compost, manure)? The "organic way" is basically very sound, because organic matter (in the form of humus) can add nutrients to the soil and markedly improve soil physical condition (filth, waterholding capacity) and nutrientholding ability. Unfortunately, some misleading and illusory claims on both sides of the issue cause a lot of confusion.
Chemical fertilizers supply only nutrients and exert no beneficial effects on soil physical condition. Organic fertilizers do both. However, compost and manure are very low-strength fertilizers; 100kg of 10-5-10 chemical fertilizer contains about the same amount of NP-K as 2000 kg of average farm manure. The organic fertilizers need to be applied at very high rates (about 20,000-40,000 kg/ha per year) to make up for their low nutrient content and to supply enough humus to measurably imrpove soil physical condition.
Overwhelming evidence indicates that chemical and organic fertilizers work best together. A study at the Maryland Agricultural Experiment Station (U.S) showed a 2033 percent yield increase when chemical fertilizers and organic matter were applied together, compared with applying double the amount of either alone.
Most small farmers will not have access to enough manure or other organic matter to cover more than a small portion of their land adequately. When supplies are limited, they should not be spread too thinly and are often most effective on high-value crops (such as vegetables) grown intensively on small plots.
Manure
Fertilizer value: Animal manure is an excellent source of organic matter, but relatively low in nutrients. The actual fertilizer value depends largely on the type of animal, quality of the diet, kind and amount of bedding used, and how the manure is stored and applied. Poultry and sheep manure usually have a higher nutrient value than horse, pig or cattle manure. Constant exposure to sunlight and rainfall will drastically reduce manure's fertilizer value.
On the average, farm manure contains about 5.0 kg N, 2.5 kg P2O5, and 5.0 kg K2O per metric ton (1000 kg), along with various amounts of the other nutrients. This works out to a 0.5-0.25-0.5 fertilizer formula. (See the chemical fertilizer section for an explanation of how fertilizer ratios are determined if this is confusing.) BUT only about 50 percent of the N, 20 percent of the P, and 50 percent of the K is readily available to plants during the first month or two, because most of the nutrients are in the organic form which first has to be converted to the available inorganic form by soil microbes. This does mean, however, that manure has good residual fertilizer value.
Farm manure is low in phosphorus: It tends to have too little available P in relation to available N and K. If used as the sole source of fertilizer, some experts recommend fortifying it with 25-30 kg of single superphosphate (0-20-0) per 1000 kg of manure. This also helps reduce the loss of N as ammonia. However, it is more convenient and effective to apply chemical fertilizer directly to the soil instead of attempting to mix it with the manure. Manure as a source of micronutrients: When livestock such as pigs and chickens are fed largely on nutritionallybalanced commercial feeds, their manure may be a particularly good source of micronutrients if applied at a high rate. Manure from animals fed mainly on local vegetation is likely to have a lower micronutrient content. How to store manure: It is best to store it under a roof or in a covered pit, but manure can be stored in piles with steep sides to shed water and good depth to reduce leaching losses by rain.
Guidelines for applying manure:
- Manure is best applied a couple of weeks to a few days before planting. If applied too far in advance, some N may be lost by leaching. To avoid "burning" the crop seeds and seedlings, fresh manure should be applied at least a couple of weeks in advance; rotted manure is unlikely to cause damage.
- Manure containing large amounts of straw may actually cause a temporary N deficiency unless some fertilizer N is added.
- Manure should be plowed, disked or hoed under soon after application. A delay of just one day may cause a 25 percent loss of N as ammonia gas.
- Rates of 20,000-40,000 kg/ha are generally recommended, but limit poultry and sheep manure to about 10,000 kg/ha since it is more likely to cause "burning". This works out to about 2-4 kg/sq. m ( 1 kg/sq. m for poultry and sheep manure).
- If quantities are limited, farmers are better off using moderate rates over a larger area rather than a high rate on a small area.
- Manure also can be applied in strips or slots centered over the row if farmers are willing to make the extraeffort involved. This is a good way to use scarce amounts. Fresh manure may burn seeds or seedlings if not mixed well with the soil.
Compost
As with manure, large amounts are needed to improve soil physical condition or supply meaningful amounts of nutrients. Compost-making takes a tremendous amount of labor and is seldom feasible for anything but small garden plots. (For more information on compost, refer to the PC/ICE Soils, Crops, and Fertilizer Use manual.)
Other Organic Fertilizers
Blood meal and cottonseed meal have much higher N contents than manure and compost, and contain other nutrients as well. However, they are valued as animal feeds and are likely to be too expensive. Bone meal (15-20 percent P2O5 ) makes P available very slowly and is also expensive.
The hulls of rice, cotton-seed, and peanuts have virtually no nutrient value, but can be used as mulches or to loosen up clayey soils on small plots. They may cause a temporary N tie-up.
Green Manure CropsSee Chapter 4, page 94.
Chemical fertilizers[edit | edit source]
Chemical fertilizers (also called "commercial or "inorganic" fertilizers) contain a much higher concentration of nutrients than manure or compost, but lack their soil-improving qualities.
Few farmers will have enough organic fertilizer to cover more than a fraction of their land adequately, so chemical fertilizers are usually an essential ingredient for improving yields quickly. Despite their ever-increasing cost, they can still frequently return good value if correctly used.
Types of Chemical Fertilizers
For soil application, granules are the most commonly used form. They usually contain one or more of the "Big Three" (N, P, K), varying amounts of sulfur and calcium (as carriers), and very low or nonexistent amounts of micronutrients.
The fertilizers can be either simple mechanical mixes of two or more fertilizers or an actual chemical combination with every granule identical in nutrient content.
How to Read a Fertilizer Label
All reputable commercial fertilizers carry a label stating their nutrient content, not only of NP-K, but also of any significant amounts of sulfur, magnesium, and micronutrients. The Three Number System: This states the content of NP-K in that order, usually in the form of N, P2O5, and K2O. The numbers always refer to percent. A 12-24-12 fertilizer contains 12 percent N, 24 percent P2O5, and 12 percent K2O which is the same as 12 kg N, 24 kg P2O5 and 12 kg K2O per 100 kg. A 0-21-1 fertilizer has no nitrogen or potassium, but contains 21 percent P2O5. Here are some more examples:
- 300 kg 16-20-0 contain 48 kg N, 60 kg P2O5 0 kg K2O
- 250 kg 12-18-6 contain 30 kg N, 45 kg P2O5 0 kg K2O
The Fertilizer Ratio
The fertilizer ratio refers to the fertilizer's relative proportions of K, P2O5 and K2O. A 12 24-12 fertilizer has a 1:2:1 ratio and so does 6-12-6; it would take 200 kg of 6-12-6 to supply the same amount of N-P-K as 100 kg of 12-24-12. Both 15-15-15 and 10-10-10 have a 1:1:1 ratio. N, P2O5, K2O versus N, P, K: Note that a fertilizer's N content is expressed as N, but that the P and K content is usually expressed as P2O5 and K2O. This system dates back to the advent of chemical fertilizers in the 19th Century and is still used by most countries, although a few have switched to an NP-K basis. A fertilizer recommendation given in terms of "actual P" and "actual K", refers to the new system; check the fertilizer label to see if the nutrient content is given as N-P2O5 - K2O or as N-P-K.
The formulas below show how to con vert between the 2 systems:
P X 2.3 = P2O5P2O5 X 0.44 = PK X 1.2 = K2OK2O X 0.83 = K
For example, a fertilizer with a 14-14-14 N-P2O5-K2O label would be labeled 14-6.2-11.6 on an N-P-K basis. Likewise, if a fertilizer recommendation calls for applying 20 kg of "actual P" per hectare, it would take 46 kg (i.e. 20 2.3) of P2O5 to supply this amount. Table 6 gives the nutrient content of common fertilizers. (Refer to pages 74-78 of PC/ICE'S Soil, Crops and Fertilizer Use manual for more detailed information.)
Basic guidelines for applving chemical fertilizers[edit | edit source]
Nitrogen
When fertilizing maize, sorghum, and millet, one-third to one-half of the total N should be applied at planting time. This first application will usually be in the form of an N-P or N-P-K fertilizer. The remaining N should be applied in one to two sidedressings (fertilizer applications made along the row while the crop is growing) later on in the growing season when the plants' N usage has increased. A straight N fertilizer like urea (4546 percent N), ammonium sulfate (2021 percent N) or ammonium nitrate (33-34 percent N) is recommended for sidedressings. When one sidedressing is to be made, it is usually best applied when the crops are about knee-high (25-35 days after plant emergence in warm areas). On very sandy soils or under high rainfall, two sidedressings may be needed and are best applied at the knee-high and flowering stages.
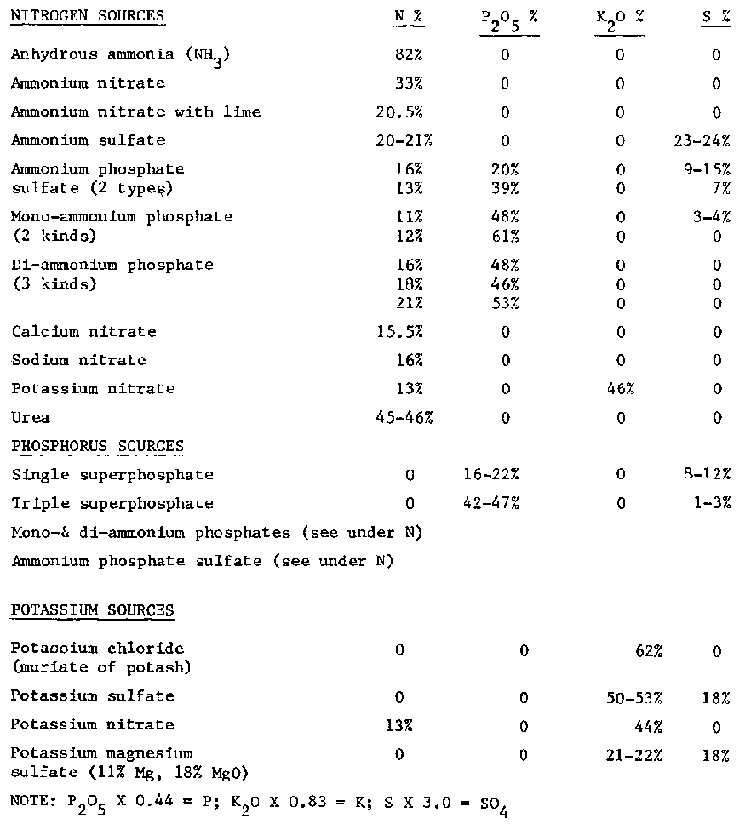
Table 6 Composition of common fertilizers
Table 6 COMPOSITION OF COMMON FERTILIZERS
|
NITROGEN SOURCES |
N % |
P2O5 |
K2O % |
S % |
|
Anhydrous ammonia (NH3) |
82% |
0 |
0 |
0 |
|
Ammonium nitrate |
33% |
0 |
0 |
0 |
|
Ammonium nitrate with lime |
20.5% |
0 |
0 |
0 |
|
Ammonium sulfate |
20-21% |
0 |
0 |
23-24 |
|
Ammonium phosphate |
16% |
20% |
0 |
9-15 |
|
sulfate (2 types) |
13% |
39% |
0 |
7 |
|
Mono-ammonium phosphate |
11% |
48% |
0 |
3-4 |
|
(2 kinds) |
12% |
61% |
0 |
0 |
|
Di-ammonium phosphate |
16% |
48% |
0 |
0 |
|
(3 kinds) |
18% |
46% |
0 |
0 |
|
21% |
53% |
0 |
0 | |
|
Calcium nitrate |
15.5% |
0 |
0 |
0 |
|
Sodium nitrate |
16% |
0 |
0 |
0 |
|
Potassium nitrate |
13% |
0 |
46% |
0 |
|
Urea |
45-46% |
0 |
0 |
0 |
|
PHOSPHORUS SOURCES |
||||
|
Single superphosphate |
0 |
16-22% |
0 |
8-12 |
|
Triple superphosphate |
0 |
42-47% |
0 |
1-3 |
|
Mono-& all-ammonium phosphates (see under N) |
||||
|
Ammonium phosphate sulfate (see under N) |
||||
|
POTASSIUM SOURCES |
||||
|
Potassium chloride |
0 |
0 |
62% |
0 |
|
(muriate of potash) |
||||
|
Potassium sulfate |
0 |
0 |
50-53% |
18% |
|
Potassium nitrate |
13% |
0 |
44% |
0 |
|
Potassium magnesium |
0 |
0 |
21-22% |
18% |
|
sulfate (11% Mg, 18% MgO) |
NOTE: P2O5 X 0.44 = P; K2O X 0.83 = K; S X 3.0 = SO4
Where to Place Nitrogen Fertilizer
As an N-P or NP-K Fertilizer: See the section on phosphorus below. As an N Sidedressing: There is no need to place a straight N fertilizer as deep as with P and K, since rainfall will carry the N downward into the root zone. Work it in 1.0-2.0 cm to keep the fertilizer from being carried away by surface water flow. Urea should always be worked in to avoid loss of N as ammonia gas.(The same is true for all ammonium N fertilizers when soil pH is above 7.0')The best time to sidedress is right before a weeding (cultivation)- the cultivator or hoe can then work it into the soil a bit.
Nitrogen can be placed in a continuous band along the crop row 20 cm or more out from the plants. Crops with spreading root systems like maize, sorghum, and millet can be sidedressed mid-way between the rows with no loss of effect. There is no need to broadcast the N to encourage better distribution, because it will spread outward as it moves downward through the soil. Avoid spilling fertilizer on the crop leaves since it can burn them. (Fertilizer burn occurs when too much fertilizer is deposited too close to the seeds or seedlings, causing them to turn brown and lose ability to absorb water.) If time is short, every other row can be sidedressed with twice the per-row amount.
Phosphorus
Phosphorus is virtually immobile in the soil. This means that fertilizers containing P should be placed at least 7.5-10 cm deep to assure good root uptake. The roots of most crops are not very active close to the soil surface (unless some form of mulching is used) since the soil dries out so readily. For these reasons, all the P fertilizer should be applied at planting time:
- Young seedlings need a high concentration of P in their tissues for good early growth and root development.
- Phosphorus does not leach, so there is no need to make additional sidedressings.
- To be effective as a sidedressing, P would also need to be placed deep (except on a heavily mulched soil), and this might damage the roots.
NOTE: Many farmers waste money by sidedressing with N-P, N-P-K or P fertilizers after already applying P at planting. Others do not apply P until the crop is several weeks old. In either case, crop yields suffer.
How to Minimize Phosphorus Tie-up
Only about 5-20 percent of the fertilizer P that the farmer applies will actually become available to the growing crop. Application method has a big influence on the amount of tie-up that occurs. In general, farmers should not broadcast fertilizers containing P, even if they plow or hoe them into the ground. Broadcasting maximizes P tie-up by spreading the fertilizer too sparsely and exposing each granule to full soil contact. Broadcasting gives a much better distribution of P throughout the topsoil, but very high rates are needed to overcome tie-up and few small farmers can afford them. In fact, it takes about two to ten times as much broadcast P to produce the same effect as an equal amount of locally placed P. Instead, farmers should use one of the localized placement methods described below. Concentrating the fertilizer in a small area enables it to overcome the tie-up capacity of the immediate surrounding soil.
Adding large amounts of organic matter to the soil helps decrease P tie-up, but usually is not feasible on large fields. Soil pH should be maintained within the 5.57.0 range if possible. Very acid soils have an especially high P tie-up capacity. When P is applied as an N-P or N-P-K fertilizer, the N helps increase the uptake of P by the plant roots.
Placement of P Fertilizers
Continuous band method: This is the best method for the reference crops and is especially well suited to closely spaced drill plantings. The optimum location of the band is 5.0-6.0 cm to the side of the seed row and 5.0-7.5 cm below seed level. One band per row is sufficient.
How to make a band: The farmer has a couple of choices:
a. Fertilizer band applicators are available for most models of tractor drawn planters and for some animal-drawn planters. Handpushed band applicators are also available commercially. The International Institute for Tropical Agriculture (IITA) farming systems program has designed a hand-pushed model that can be built in any small workshop with welding and metal shearing capabilities. However, it is not clear from the design whether the IITA model actually places the fertilizer below the soil surface.
b. Plow or hoe methods
- The farmer can make a furrow 7.5-15 cm deep with a wooden plow or hoe, apply the fertilizer by hand along the bottom, then kick in enough soil to fill the furrow back up to planting depth. This gives a band of fertilizer running under the seeds and a little to each side. As long as there is 5.0-7.5 cm of soil separating the fertilizer and the seeds, there is little danger of burning.
- A less satisfactory method is to make one furrow at planting depth and place both seed and fertilizer in it together (the furrow should be wide so the fertilizer can be spread out and diluted somewhat). This works with maize at low to medium rates of N and K (no more than 200-250 kg/ha of 16-20-0 or 14-1414, no more than 100-125 kg ha of 18-460 or 16-48-0). Higher rates may cause fertilizer burn. Beans and sorghum are more sensitive to fertilizer burn than maize. Halfcircle Method: Works well when seeds are planted in groups ("hill planting") spaced relatively far apart on untilled ground where banding would be impractical. The fertilizer is placed in a half circle made with a machete, hoe, or trowel about 7.5-10 cm away from each seed group and 7.5-10 cm deep. This is time-consuming, but gives a better distribution of fertilizer than the hole method. Hole method: This is the least effective of the three methods, but is much better than using no fertilizer at all. It may be the only feasible method for land that has been hill planted without any prior tillage. Fertilizer is placed in hole 10-15 cm deep and 7.5-10 cm away from each seed group.
Potassium
Potassium ranks midway between N and P in terms of leaching losses. As with P, all the K can usually be applied at planting time, often as part of an N-P-K fertilizer. Where leaching losses are likely to be high (very sandy soils or very high rainfall), split applications of K are sometimes recommended.
Unlike N and P, about twothirds of the K that plants extract from the soil ends up in the leaves and stalks rather than in the grain. Returning crop residues to the soil is a good way of recycling K. Burning them will not destroy the K, but will result in the loss of their N, sulfur, and organic matter.
Some Special Advice For Furrow-Irrigated Soils
When using the band, halfcircle or hole methods on furrow irrigated soils (crops irrigated by conveying water along a furrow between each row or bed) the farmer should be sure to place the fertilizer below the level that the irrigation water will reach in the furrow. Placement below this "high water" mark enables mobile nutrients like nitrate and sulfate to move sideways and downwards toward the roots. If placed above the water line, the upward capillary movement of water will carry these mobile nutrients to the soil surface where they cannot be used. (Upward capillary movement is the same process that enables kerosene to "climb" up the wick in a lamp.)
Determining how much fertilizer to use[edit | edit source]
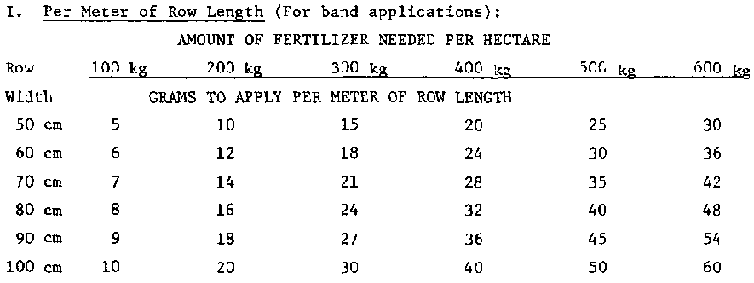
The following table can be used to determine how much fertilizer to apply per length of row (if the half-circle or hole method is used). (The formula found in PC/ICE's Soil, Crops and Fertilizer Use manual can also be used to determine this amount.)
NOTE: Rather than tell farmers to apply so many grams or ounces per length of row or per hill, convert the weight dosage to a volume dosage using a commonly available container like a tuna fish or juice can, jar lid or bottle cap.
Fertilizers vary in density, so be sure to determine the weight/ volume relationship for each type using an accurate scale.
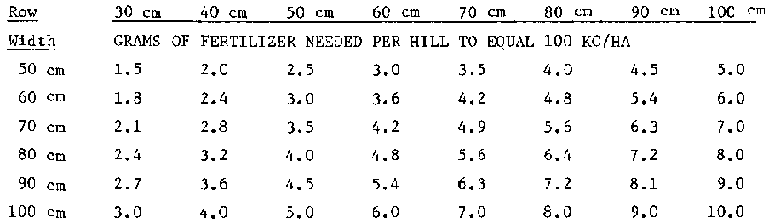
Table 7 Determining How Much Fertilizer is Needed per Meter of Row Length or per "Hill"
II. Per Hill (For half-circle or hole applications): In this case, the amount depends on the row spacing and the distance between hills in the row. The table below shows how many grams of fertilizer are needed per hill to equal a rate of 100 kg/ha. To find out how much would be needed to equal a rate of 250 kg/ha, you would multiply the table's figures by 2.5.
Distance between hills
Foliar Fertilizers
Foliar applications are best suited for micronutrients: Soluble powder or liquid fertilizers may be sold in some areas for mixing with water and spraying on the leaves. Some granular fertilizers like urea, ammonium nitrate, and di-amonium phosphate are also soluble enough for this purpose. However, only small amounts of fertilizer can be sprayed on the leaves per application without causing "burning" - this means that foliar applications are usually best suited for micornutrients of which very little is needed. Foliar applications are especially useful for applying iron, which becomes readily tied up and unavailable when applied to the soil. Although foliarapplied fertilizers take effect rapidly (within one to three days) they have much less residual value than soil applications.
N-P-K foliar fertilizer are often claimed to produce very profitable yield increases.
- Numerous trials have shown that N-P-K foliar fertilizers usually "green up" the leaves but significant yield increases are unlikely as long as sufficient N-P-K is applied to the soil. A 1976 International Center for Tropical Agriculture (CIAT) trial in Colombia did obtain a 225 kg/ha yield increase on beans by spraying them three times with a 2.4 percent solution (by weight) of mono-ammonium phosphate (11-480) even though 150 kg/ha of P2O5 had been added to the soil. (The spray contributed only about 10 kg/ha of P2O5.) However, the soil had a very high P tie-up capacity.
The soluble powder and liquid foliar fertilizers are much more expensive per unit of nutrient compared to ordinary granular fertilizers.
Numerous applications are usually needed to supply a meaningful amount of N-P-K through the leaves without risk of burning.
Some N-P-K foliar fertilizers have micornutrients included but the amounts are far too small for preventing or curing deficiencies.
How to Avoid Fertilizer "Burn"
Fertilizer "burn" occurs when too much fertilizer is placed too close to the seeds or seedlings. It is caused by a high concentration of soluble salts around the seed or roots which prevents them from absorbing water. The seeds may germinate poorly from tips downward, the seedling leaves may begin to turn brown, and the plants may die.
Guidelines for Avoiding Fertilizer Burn
- The N and K in fertilizers have a much higher "burning" ability than P. Single and triple superphosphate are very safe. Sodium nitrate and potassium nitrate have the highest burn potential per unit of plant nutrient followed by ammonium sulfate, ammonium nitrate, monoammonium phosphate (11-48-0), and potassium chloride. Di-ammonium phosphate (16-48-0, 18-46-0) and urea can injure seeds and seedlings by releasing free ammonia gas. The higher the ratio of N and K to P in an N-P-K fertilizer, the greater the likelihood of burning due to improper placement.
- When using fertilizers containing N, do not place them any closer than 5 cm to the side of the seed row when banding and 7.5 cm when using the half-circle or hole methods (see exceptions discussed under banding methods). There is little danger of burning when sidedressing growing crops with N, but avoid dropping the granules on the leaves.
- Fertilizer burn occurs more frequently on sandy soils than on clayey soils and under low moisture conditions. A heavy rain or irrigation will help carry damaging salts away if burning occurs.
Recommended fertilizer rates for the reference crops[edit | edit source]
The most profitable rate of fertilizer use for the small farmer depends on management ability, capital available, limiting factors, soil fertility level, type of crop, expected price and cost of fertilizer.
Small farmers should usually aim for maximum return per dollar spent. This means using low to moderate rates of fertilizer because crop yield response is subject to the law of diminishing returns.
Since the efficiency of fertilizer response declines as rates go up, the small farmer with limited capital is usually better off applying low to medium rates of fertilizer. He or she will end up with a higher return per dollar invested, be able to fertilize more land, and have money left over to invest in other complementary yield-improving practices.
As a farmer's capital situation improves, higher rates of fertilizer may be justified as long as investments in other worthwhile practices are not sacrificed. Another factor to consider is that fertilizer can reduce the land and labor needed to produce a given amount of crop, thus cutting costs and allowing for more diversity of production.
Some General Guidelines for Low, Medium and High Rates Of N-P-K
Keeping in mind the many factors that determine optimum fertilizer rates, Table 8 provides a very general guide to LOW, MEDIUM, and HIGH rates of the "Big Three" for the reference crops based on small farmer conditions and using localized placement of P. The "high" rates given here would be considered only low to medium by most farmers in Europe and the U.S. where applications of 200 kg/ha of N are not uncommon on maize and irrigated sorghum.
There are several important qualifications to Table 8:
- YOU MUST CONSIDER THE FERTIILITY LEVEL OF THE SOIL as well as the type of crop. A soil high in available K would need little or no fertilizer K. Most cropped soils tend to be low in N and low to medium in P, but K deficiencies are less common. Peanuts often respond better to residual P and K rather than to direct applications.
- Legumes such as peanuts, cowpeas, soybeans, pigeonpeas, mung beans, and chickpeas are very efficient N fixers if properly innoculated with the correct strain of Rhizobia bacteria or if grown on soils with a good natural population of the correct Rhizobia. In some cases, however, a starter application of 15-25 kg/ha of N has given a positive response by feeding the plants until the Rhizobia begin to fix N (about two to three weeks after plant emergence). Such responses are the exception rather than the rule and are most likely to occur on sandy soils. Beans (Phaseolus vulgaris) are not so efficient at N fixation and can use up to 50-60 kg/ha of N.
- The farmer's management ability is a vital consideration. Farmers should not be encouraged to use a high rate of fertilizer if he or she is not willing or able to use other complementary yieldimproving practices.
Table 8 General guidelines for low, medium, and high rates of N-P-K
|
LOW (Lbs./acre or kg/hectare) |
MEDIUM(Lbs./acre or kg/hectare) |
HIGH(Lbs./acre or kg/hectare) | |
|
N² |
35-55 |
60-90 |
100+ |
|
P2O5 |
25-35 |
40-60 |
70+ |
|
K2O |
30-40 |
50-70 |
80+ |
Fertilizer recommendations for specific crops[edit | edit source]
Fertilizer Response
When starting from a low yield base like 1000-1500 kg/ha, yields of shelled maize should increase by roughly 25-50 kg for each kg of N applied up to a yield of around 40005000 kg/ha. With higher rates of application, response generally falls below this ratio. Such yield increases will be obtained if:
- Other nutrients like P and K are supplied as needed, soil moisture is adequate, a responsive variety is used, and there are no serious limiting factors such as insects, diseases, weeds, soil ph, drainage, etc.
- The fertilizers are applied correctly and at the right time. If response falls velow the 25-50 level, this means that one or more serious limiting factors is present or that too high an N rate was used. Table 8 can be used as a guide, but soil should be tested whenever possible. Studies have shown that maize can use locally-placed (band, half-circle, hole) P efficiently up to about 50-60 kg/ha of P2O5. Micronutrients: Except for zinc, maize is not very susceptible to micronutrient deficiencies. Zinc deficiency can be confirmed by spraying about 20 plants with a solution of one tablespoon (15 cc) zinc sulfate in about four liters of water along with about 5 cc of liquid dishwashing detergent as a wetting agent. If zinc is the only nutrient lacking, new leaves will have a normal green color when they emerge.
Table 9
|
Zinc Source |
% Zinc |
Amount Needed |
Application Method |
|
Zinc sulfate monohydrate |
23% |
8-12 kg/ha (lbs./acre) |
mixed with planting fertilizer and locally placed |
|
Zinc sulfate heptahydrate |
35% |
6-9 kg/ha (lbs./acre) |
mixed with planting fertilizer and locally placed |
|
Zinc oxide |
78% |
|
mixed with planting fertilizer and locally placed |
|
Zinc sulfate |
23%, 35% |
350-500 grams/100 liters water plus a wetting agent |
Foliar; spray the leaves; may cause leaf burn under some conditions. |
Fertilizer Response: Sorghum will give similar fertilizer responses to maize if moisture is adequate and improved varieties are used. As always, farmers should be encouraged to test the soil first rather than rely on general recommendations.
Nutrient needs are similar to those of maize, except that sorghum is most susceptible to iron deficiency.
Iron deficiencies seldom respond well to iron applied in the soil unless special chelated (organic and more costly) forms are used to protect against tie-up. Deficiencies should be treated by spraying the plants with a solution of 2-2.5 kg of ferrous sulfate dissolved in 100 liters of water along with sufficient wetting agent to assure uniform leaf coverage. Begin spraying as soon as symptoms appear; the plant may need several applications during the growing season on severely deficient soils.
Sorghum seeds and seedlings are more sensitive to fertilizer burn than maize. If more than one grain harvest is to be taken from one planting, all the P and K should be applied at planting along with about 30-50 kg/ha of N. Another dressing of 30-50 kg/ha of N should be applied about 30 days later. After the first harvest, apply an additional 30-50 kg/ha 25-30 days later.
Fertilizer Response: Low soil moisture is a major factor limiting fertilizer response. Traditional varieties are usually less responsive. Studies in India by ICRISAT showed that improved pearl millet varieties were responsive to N rates as high as 160 kg/hectare under adequate moisture, but that traditional types seldom responded well above the 4080 kg/hectare range. The N-P-K rates in Table 8 can be used as a guide, taking into consideration the moisture and variety factors.
Fertilizer Response: Peanuts tend to give rather unpredictable responses to fertilizer and offer respond best to residual fertility from previous applications to other crops in the rotation.
Nitrogen and Nodulation: If the right strain of Rhizobia bacteria is present, peanuts can ordinarily satisfy their own N requirements. There are two exceptions:
- If poorly drained portions of the field become waterlogged temporarily, the Rhizobia may die off and the plants begin to turn yellow. An application of 20-40 kg/ha of N may be needed to carry the plants through until the bacteria become reestablished in several weeks.
- In some cases (mainly on lightcolored, sandy soils), 20-30 kg/ ha of N applied at planting has seemed to help the plants establish themselves until the Rhizobia begin to fix N about three weeks after emergence. This is not widely recommended.
To check for proper nodulation, carefully remove the roots of plants at least three weeks old and look for clusters of fleshy nodules (up to the size of small peas) especially around the tap root. Slice a few open - if they are reddish inside, this shows they are actively fixing N.
Seed innoculation is normally not necessary if peanuts are sown on land that has grown peanuts, cowpeas, lima beans, mung beans or crotalaria within the past three years. Commercial innoculant is a dark-colored, dried powder which contains the living Rhizobia and comes in a sealed packet. Seed is placed in a basin and moistened with water to help the innoculant stick (adding a bit of molasses helps, too). The correct amount of innoculant is mixed with the seed, and planted within a few hours. Exposing the seed to sunlight can kill the bacteria.
Phosphorus and Potassium: Because peanuts have an unusually good ability to utilize residual fertilizer from preceding crops, they do not respond well to direct applications of P and K unless levels are very low. In fact, there is good evidence that high K levels in the podding zone can increase the number of pops (unfilled kernels) due to decreased calcium availability.
Calcium: Peanuts are one of the few crops having a high Ca requirement. Light green plants plus a high percentage of pops may indicate Ca hunger. Calcium does not move from the plant to thepods; rather, each pod has to absorb its own requirement. Gypsum (calcium sulfate) is used to supply Ca to peanuts because it is much more soluble than lime and has no effect on soil pH (using lime to supply Ca can easily raise the pH too much). The usual application where deficiencies exist is 600-800 kg/ ha of dry gypsum applied right over the center of the crop row (it will not "burn") in a band 40-45 cm wide any time from planting until flowering.
Gypsum also supplies sulfur. Micronutrients: Boron and manganese are the ones most likely to be deficient (see Table 5). Boron can be toxic if applied at rates much above those given in Table 10 especially when banded.
Table 10 Suggested Boron (B) and Manganese (MN) Rates For Peanuts on Deficient Soils
|
Material |
% B or Mn |
Amount Needed |
How Applied |
|
Borax |
11% B |
5-10 kg/ha |
Mixed with fungicide dusts for leafspot or mixed with gypsum. Do not locally place boron or injury may result. |
|
Solubor |
20% B |
|
Spray plants |
|
Manganese sulfate |
26-28% Mn |
15-20 kg/ha |
Banded with row fertilizer at planting |
|
Soluble manganese sulfate |
26-28% Mn |
5 kg/ha |
Spray on plant leaves; use wetting agent. |
|
Manganese sulfate |
26-28% Mn |
15 kg/ha |
Dust the plants with the finely ground form |
Beans (Kidney Beans)
Nitrogen: Beans are less efficient N fixers than peanuts or cowpeas and recommended N rates usually fall in the range of 40-80 kg/ha N. In a 1974 CIAT trial in Colombia, 40 kg/ha N increased yields to 1450 kg/ha compared to 960 kg/ha with no N. It was found that acid forming fertilizer N sources such as urea and ammonium sulfate could increase the chances of aluminum and manganese toxity if banded near the row on very acid soils. It was recommended that the N be spread out more in these cases.
Phosphorus: Beans have a high P requirement, and this is often the major limiting nutrient, especially on soils with a high capacity to tie up P. A 1974 CIAT trial on such a soil resulted in yields of 700 kg/ha without P and 1800 kg/ha when 200 kg/ ha of P2O5 was banded along the row. Such high rates of P may be needed on soils with serious P tie-up problems. Under such conditions, it might take 10 times this amount to give the same effect if broadcast.
Potassium deficiencies are uncommon in beans.
Magnesium deficiency may occur in very acid soils or those high in Ca and K. It can be controlled by applying 100-200 kg/ha of magnesium sulfate or 20-30 kg/ha or magnesium oxide to the soil. If the soil needs liming, using dolomitic limestone (2045 percent Mg) will solve the problem. Dolomitic limestone and magnesium oxide should be broadcast and plowed or hoed under before planting. Magnesium sulfate (epsom slats) can be band-applied or sidedressed. A foliar application of one kg magnesium sulfate per 100 liters water can be tried on established crops.
Micronutrients: Beans are most susceptible to manganese, zinc, and boron deficiencies (see Table 5). Varieties differ in their susceptibilities. Zinc rates: As for maize. Manganese: As for peanuts. Boron: 10 kg/ha of borax banded with the row fertilizer at planting or 1 kg Solubor (20 percent B) per 100 liters of water sprayed on plants. Manganese toxicity is sometimes a problem on very acid soils, especially if they are poorly drained. Symptoms are easily confused with those of zinc and magnesium deficiency. Beans are also very sensitive to aluminum toxicity which occurs below a pH of 5.2-5.5, and liming the soil is the only control. If aluminum toxicity is severe, plants may die shortly after emergence. In more moderate cases, the lower leaves become uniformly yellow with dead margins, the plants become stunted, and yields can be lowered dramatically.
Well-nodulated cowpeas do not respond to N applications, although a starter dosage of 10 kg/ha N sometimes shows results.
Liming[edit | edit source]
Soils with a pH below 5.0-5.5 (depending on the soil) can adversely affect crop growth in four ways:
- Aluminum, manganese, and iron toxicities: These three elements increase in sulubility as soil pH drops and may actually become toxic to plants at pH's below 5.0-5.5. Beans are especially sensitive to aluminum toxicity which is the crop's biggest yield limiting factor in some areas. Many soils labs routinely test for soluble aluminum levels in very acid samples. Manganese and iron toxicities can be serious, too, but usually are not a problem unless the soil is also poorly drained.
- Very acid soils are usually low in available P and have a high capacity to tie up added P by forming insoluble compounds with iron and aluminum.
- Although very acid soils usually have enough calcium to supply plant needs (except for peanuts), they are likely to be low in magnesium and available sulfur and molybaenum.
- Low soil pH depresses the activities of many beneficial soil microbes such as those that convert unavailable N, P, and S to available mineral forms.
Maize and cowpeas may tolerate soil acidity in the pH 5.0-5.5 range depending on the soil's soluble aluminum content. Sorghum is somewhat more tolerant than maize to soil acidity. Peanuts commonly do well down to pH 4.8-5.0 since they have comparatively good aluminum tolerance. Beans are the most sensitive of the reference crops to soil acidity, and yields usually decline below a soil pH of 5.3-5.5.
Where are Acid Soils Likely To be Found?
Soils in higher rainfall areas are likely to be slightly acid to strongly acid since a good deal of calcium and magnesium may have been leached out over time by rainfall. Those of drier regions are likely to be alkaline or only slightly acid due to less leaching.
Continual use of nitrogen fertilizers, whether chemical or organic will eventually lower soil pH enough to require liming. Calcium nitrate, potassium nitrate, and sodium nitrate are the only exceptions and are usually too expensive or unavailable.
How to Tell if Liming is Needed
Soil pH can be measured fairly accurately right in the field with a liquid indicator kit or a portable electric tester. These are useful for troubleshooting but have two drawbacks:
- Soil ph is not the sole criteria for determining if liming is needed. The soil's content of soluble aluminum (called "exchangeable" aluminum) is probably even more important, and the portable pH kits cannot measure this. A soil with a pH of 5.0 or even lower might still be satisfactory for the growth of most crops if its exchangeable aluminum content is low. On the other hand, another soil with a pH of 5.3 might need liming because of too much aluminum. Only the soils lab can tell for sure.
- The amount of lime needed to raise soil pH one unit varies greatly with the type of soil involved. One soil may require 810 times more lime than another to achieve the same rise in pH even though both have the same initial pH. The amount of lime needed depends on the soil's amount of negative charge which varies with its texture, type of clay minerals, and amount of humus. Only the soils lab can determine this.
Calculating the Amount of Lime Needed
Whether using the lab's or other recommendations, adjustments still must be made for the fineness, purity, and neutralizing value of the material used:
- Neutralizing value: On a more pure basis, here are the neutralizing values of four liming materials:
|
Material |
Neutralizing Value (compared to limestone) |
|
Limestone (calcium carbonate) |
100 percent |
|
Dolomitic limestone (Ca + Mg carbonate) |
109 percent |
|
Hydrated lime (calcium hydroxide) |
136 percent |
|
Burned lime (calcium oxide) |
179 percent |
This means that 2000 kg of burned lime has about the same effect on pH as 3580 kg of limestone of equal purity (2000 kg x 1.79 = 3580 kg).
- Fineness of material greatly affects the speed of its reaction with the soil. Even finely ground material may take two to six months to affect soil pH.
- Purity: Unless the material has a label guarantee, it is difficult to judge purity without a lab analysis.
How, When, and How Often To Lime
- Lime should be broadcast uniformly over the soil and then thoroughly mixed into the top 15-20 cm by plowing or hoeing. Harrowing alone will only move the material down about half this distance. A disk plow or moldboard plow should be used, not a wooden or chisel plow. If spreading lime by hand, the amount should be divided in half and one portion applied lenghtwise and the other widthwise. Wear a mask hydrated (slaked) lime and burned lime can cause severe burns.
- Whenever possible, a dolomitic form of liming material should be used to avoid creating a magnesium deficiency.
- Liming materials should be applied at least two to six months ahead of planting, especially if the material is not well ground.
- Liming may be needed every two to five years on some soils, especially if high rates of nitrogen fertilizers, manure or compost are used. Sandy soils will need more frequent liming than clayey soils since they have less buffering capacity, but sandy soils also will require lower rates.
DO NOT OVERLIME'
- Never raise the pH of soil above 6.5 when liming.
- Never raise the pH by more than one full unit at a time (i.e. from 4.6 to 4.6, etc.). It is only necessary to raise the pH up to 5.5-6.0 for good yields of an aluminum sensitive crop like beans.
Overliming can be worse than not liming at all for several reasons:
- Raising the pH above 6.5 increases the likelihood of micronutrient deficiencies, especially iron, manganese, and zinc; molybdenum is an exception.
- Phosphorus availability starts declining once pH rises much above 6.5 due to the formation of relatively insoluble calcium and magnesium compounds.
- Liming stimulates the activity of soil microbes and increases the loss of soil organic matter by decomposition.
Water management[edit | edit source]
Water Needs of The Reference Crops
Relative Differences: Millet has the best drought resistance of the three cereals, followed by sorghum, and then maize. Of the pulses, cowpeas and peanuts are superior to common beans in this respect. Critical Water Demand Periods: The critical water demand period for all the reference crops in terms of both yield effect and maximum usage occurs from flowering time through the soft-dough grain stage. Under low humidity and high heat, total water usage (soil evaporation and plant transpiration) may reach 910 mm per day during flowering and early grain filling. Effect of Moisture Stress on Yields: Crops can often overcome the effects of moisture stress occurring early in the season, but yields can be markedly lowered if it occurs during flowering and grain filling. With maize one to two days of wilting during tasseling time can lower yields by up to 22 percent and six to eight days by 50 percent.
Symptoms of Moisture Stress
- Maize, sorghum, and millit will begin to roll their leaves up lengthwise, and the plants will turn a bluish green color. The lower leaves will often dry up and die. (This is referred to as "firing" and is really a drought-induced nitrogen deficiency.)
- The pulse crops will also turn a bluish-green and Their leaves will wilt as stress increases. "Firing" may also occur.
Factors Influencing the Likelihood of Moisture Stress:
- Rainfall pattern and quantity: See the section on rainfall in Chapter 2.
- Soil texture: This has a big influence on a soil's water storage capacity. Clay loams and clay soils can hold twice as much available water per foot of depth as sandy soils.
- Soil Depth: Deep soils can store more water than shallow soils and allow greater rooting depth for utilizing it.
- Soil Slope: Much water can be lost by runoff on sloping soils.
- Temperature, Humidity, and Wind: The higher the temperature and wind and the lower the humidity, the greater the rate of crop moisture use and soil evaporation losses.
Keeping Rainfall Records
Since rainfall amount and distribution have such a great effect on crop yields, it is very useful to keep rainfall records at various locations in your work area. The more progressive client farmers should be encouraged to keep their own records. Judging Rainfall: Showers that produce less than 6 mm usually contribute little moisture to the crop since they do not penetrate the soil very deeply and are quickly evaporated. For example, 5 mm of rainfall will penetrate only about 20 mm into a dry clayey soil and 40 mm into a dry sandy soil.
Improving Water Use Efficiency
In areas with short rainy seasons, the use of early maturing varieties is a valuable tactic. Planting dates should be timed so that likely moisture stress periods do not coincide with critical crop stages such as pollination.
One study in Kenya showed a yield decrease of 5-6 percent for each day's delay in maize planting after the start of the rains (in an area with a short season). In areas having wet seasons of adequate length, but with periods of moisture stress, some extension services recommend planting two or more varieties with different maturities to lower the risk of total crop failure.
On sloping soils, soil conservation measures such as terracing or ditch-and-bank systems will significantly improve water retention in addition to reducing soil losses. Weed control both during and between crops will cut water use. In semiarid areas such as the Sahel, deep plowing should be avoided if the subsoil is moist. Fertilizer use will increase moisture use efficiency by encouraging deeper rooting. However, crops cannot utilize as much fertilizer (especially N) when water is a limiting factor.
Optimum plant populations are usually lower under conditions of low rainfall and probable moisture stress.
Mulching the soil surface with a 5.0-7.5 mm layer of crop residues can substantially increase yields in drier areas.
Guidelines for Improving Water Use Efficiency Under Furrow Irrigation
To avoid falling behind in crop irrigation needs, the soil should be pre-irrigated to the full depth of maximum expected root development before planting the crop. Moisture stored in the subsoil is usually safe from evaporation losses unless the soil cracks upon drying. Leaching losses will be negligible if the correct amount is applied since only excess water moves downward by the force of gravity - the rest is held by the soil pores.
Frequent, shallow irrigation should be avoided since it increases evaporation losses and limits the depth of root growth. Shallow irrigation encourages the buildup of harmful salts in dry climates, and frequent irrigation favors the spread of fungal and bacterial diseases. However, irrigation may have to be fairly frequent in the initial stages of crop growth until the plants have been able to put their roots down sufficiently.