UV Water Disinfection
Background[edit | edit source]
884 million people worldwide currently live without clean water or have an unsafe way to clean their water.[1] Developing communities have by far the least access to clean water and the most difficult time finding working water treatment systems. Many communities in the developing world have either second-rate disinfection systems for the community water, or they must rely on household disinfection systems. Boiling water is still the most widely used household disinfection method.[2] It is, however, the most health hazardous and most energy consuming, due to indoor smoke pollution from wood burning as the power source.[3] Though they are less energy consuming, chemical disinfectants, such as chlorine, are also an undesirable water treatment method because of the unfamiliar taste.[4] Therefore, in order to treat water for use in the developing world, a safe, inexpensive, and chemical-free approach can be used.
History[edit | edit source]
The disinfection potential of UV light was discovered well before the first UV disinfection reactor was created. In 1877, "Downes and Blunt discovered germicidal properties of sunlight," and there began both solar disinfection and UV lamp disinfection. The UV lamp was developed soon after in 1901 by the discovery that mercury could be used in a lamp to generate light. The quartz housing that is still used today was included 5 years later in 1906.[5]
Europe is known for initiating new treatment technologies, and was the first to implement UV disinfection into a drinking water treatment plant in Marsailles, France. After implementation, the link between the pathogens in the water and the treatment itself was discovered. Gates discovered that the pathogens absorbed the UV light when exposed to it. In the 1950's, Switzerland and Austria used germicidal UV lamps for drinking water treatment, starting the use of the current technology.[5] UV disinfection has been used throughout the past few decades, with most treatment systems found within Europe.[4] By 2000, UV treatment started to become a viable treatment option in the US, and now many small treatment plants use UV disinfection as well as an increasing amount of higher-flow plants, such as Seattle. Many wastewater treatment plants have been using UV disinfection for tertiary treatment before discharge. [5]
Theory[edit | edit source]
Disinfection[edit | edit source]
Disinfection is a method to inactivate pathogens, and there are three main categories when dealing with the disinfection of water: chemical, physical, and photochemical. Chlorine, Bromine, and Ozone are three commonly used chemical disinfectants. All three have disinfection byproducts which are harmful to either human contact or to the atmosphere. Physical disinfection consists of filtration, and photochemical disinfection consists of UV disinfection or SODIS (solar disinfection).[6][7]
Pathogen Inactivation[edit | edit source]
UV light disinfects most efficiently in the UV-C range of 100-280nm.
Germicidal UV (UV-C) has a very low spectral irradiance, indicating that it is difficult to utilize germicidal UV rays from the sun.[8] Therefore, to obtain effective and efficient disinfection, germicidal UV must be obtained from a UV-C lamp.

Mercury arc lamps, which are most commonly used, transmit at 254nm.[6] At 254 nm, the UV lamp transmits at 95% efficiency, so 95% of the UV-C rays will be absorbed.[9] Efficiency of light transmittance from the UV lamps are also affected by lamp characteristics, low or medium pressure lamp, the quartz housing variations, ambient conditions, and electricity availability.[6]
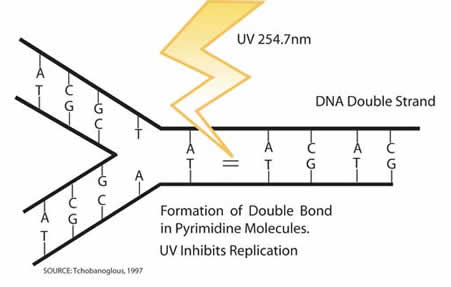
In order to disinfect the water, the UV-C rays inactivate pathogens by destroying bonds in their DNA.
After their bonds are destroyed, the DNA can no longer replicate, rendering the pathogens inactive. Complete inactivation of pathogens is dependent on receiving their lethal dose.[6]

The lethal dose is calculated based on how much energy is needed to get into the DNA of a microorganism. UV dose is dependent on both the surface area of water that is in contact with the UV rays and the energy of UV light transmitted. It is important to first calculate the percent of UV transmittance through the mircoorganisms in order to scale the dose accordingly. The percent UV transmittance is calculated using Beer's Law.[5]
Beer's Law: A
In Beer's Law, I is equal to intensity of light transmitted in [mW/cm2], I0 is equal to the intensity of incident light in [mW/cm2], and A is the UV absorbance at a certain wavelength and pathlength and is unitless.The dose is then calculated by integrating the intensity of transmitted UV light over the exposure period.[5] UV dose is generally expressed in either [mJ/cm2] or [mW-s/cm2].A dose of 40 mJ/cm2 is strong enough to obtain a 5 log kill of E. coli.[10] The national and international standard for drinking water is 38 mJ/cm2, as specified by both the American National Standards Institute and the National Sanitation Foundation International.[11] This dosage will obtain a sufficient reduction in pathogens for safe drinking water from clear water source, but will not disinfect to the full 5 log kill of E. coli.
Log kill, or log inactivation, demonstrates the microbial response to UV-C light, and is the amount of pathogen inactivation that has occurred. Log inactivation can be modeled by the following equation:
0(N_0/N)
Where N is the concentration of targeted pathogens after exposure to UV-C light, and N0 is the initial target pathogen concentration. Disinfection is also affected by the initial turbidity of the water. Water must have less than 1.0 NTU to achieve sufficient pathogen inactivation.[6]
Unlike chemical disinfection, there is no residual disinfection with UV-C light. The dosage must be high enough to eliminate the possibility of photorepair of the pathogens. When a pathogen photorepairs, it repairs the pyrimidines in the DNA in the presence of light, allowing itself to replicate again. Microorganisms can also dark repair themselves without the help of light if the UV dosage is not strong enough. [5]
Reactor Configurations[edit | edit source]
The configuration of the water treatment reactor has a significant effect on efficiency of disinfection. Generally, the UV lamps are parallel to water flow in circular channels and are either perpendicular or diagonal to the water flow for rectangular channels.[5] A treatment reactor should be designed for maximum UV exposure to the water without allowing for too much UV light to be absorbed by the reactor walls.[5]
There are two basic types of ideal reactor configurations that can be followed: the plug flow reactor (PFR) and the continuous stirred-tank reactor (CSTR). PFRs operate like an assembly line, treating each parcel of water at a time.
CSTRs mix the entire volume of water instantaneously.
Ideally, a reactor should be as plug-flow as possible for UV disinfection.[5][12] Plug flow reactors can allow for very efficient pathogen elimination.[13] By using a more plug-flow reactor with baffles included, dead space is minimized and the effluent will be sufficiently disinfected at a much faster rate than with a CSTR.[5][12]
-
Cumulative effluent distribution of an ideal PFR
-
Exit age distribution of an ideal PFR
-
Cumulative effluent distribution and exit age distribution of an ideal CSTR
UV Disinfection in Developing Countries[edit | edit source]
UV Waterworks: Community-sized UV Disinfection[edit | edit source]
The most widely used UV disinfection device within the developing world today is the UV Waterworks system.

UV Waterworks was created by Dr. Ashok Gadgil of Lawrence Berkeley National Laboratories and his research group in 1993 as a response to a cholera outbreak in India.[14][10]

After field tests in India and redesigning to meet their needs better,[15] the technology has been in use since 1998 and is promoted under WaterHealth International.[16] The UV Waterworks system can be used for approximately 1000 people, and can last for an estimated 15 years with proper operation and maintenance.[14] The reactor works fairly quickly, with a fluid detention time of 12 seconds. [10] The quickness of the UVWaterworks system is a definite advantage to other water treatment systems, which can often take minutes or even hours to treat. Its ability to disinfect up to 5-log reduction of E-coli helped get it certified by the California Department of Health Services. [10][16]
UV Waterworks is an inexpensive way for communities to get water, with an initial cost of $300 and approximately $0.10/year for a person treating 10 liters of water per day.[14][17] This disinfection system needs maintenance every three month and is very easy to operate, although it relies on having a constant source of electricity.[10]
Point-of-Use UV Disinfection[edit | edit source]
The UV Tube is another UV disinfection system designed for use in the developing world. This device is a point-of-use system, meaning it is for household use and water is not transported after treatment. Developed by Dr. Lloyd Connelly of UC Berkeley in the 1990's, the UV Tube has been implemented in Haiti, Mexico, and Sri Lanka.[18]
Also implemented in Mexico is the UVeta, which was developed by Niparajá A.C and started use in 2006.[19] The UVeta is a point-of-use system, similar to the UV Tube.
Research on point-of-use UV disinfection has been and continues to be conducted at the University of Colorado at Boulder. Dr. Karl Linden headed a research project on a point-of-use UV disinfection system using an aluminum reactor.[20]
-
The UV Tube
-
UVeta point-of-use system
-
Reactor developed at CU Boulder
Make a UV Disinfection Reactor[edit | edit source]
Materials[edit | edit source]
To make a small UV disinfection system with items available in developing countries, the following items are needed:
- Low Pressure (LP) or Medium Pressure (MP) UV bulb - may not be locally available[5]
- Quartz sleeve[5]
- Reactor housing - can use aluminum can for outside and aluminum can or plastic rings for baffles[20] or can use pipes to house UV lamp[21]
- Electrical ballast - to regulate power supply[5]
- Plastic tubing (use as needed)
- Pipe adaptors (use as needed)- for tubing to reactor connection
- Plastic bucket to house water
- Epoxy
Although LP UV lamps are less expensive than MP UV lamps, MP UV lamps use a far lower dosage to obtain the same amount of inactivation as an LP UV lamp. Also, the MP UV lamps are significantly better at inactivating adenoviruses.[22]
Construction[edit | edit source]

Construction for each UV disinfection system is material-dependent and size-dependent. For a household-size point-of-use system, construction must include the attachment of the UV reactor to the bucket and sealing any areas where there may be leaks. Whatever is being used for the reactor housing must include an opening for the UV bulb, and must be able to allow water to flow in from the bucket and out to another bucket. Holes will have to be drilled in the bucket and the reactor housing, and will require the use of a power drill.
Use[edit | edit source]
UV disinfection systems require a constant source of electricity. UV lamps used range from using 3W[20] to 50W[10] of electricity. The UV lamp must be running continuously for the disinfection to be complete.
Operation and Maintenance of System[edit | edit source]
Operation of a UV disinfection system, regardless of its size, must be powered by a source of electricity. There is no need for a skilled operator, which greatly reduces the operation cost for UV disinfection[10] Turbidity and color should be monitored regularly, if possible.[11]
Larger, community-scale systems like the UV Waterworks should undergo maintenance every 3 months, and smaller point-of-use systems should be maintained as needed.[10] Although maintenance schedules and protocols are infrequently if ever followed in most developing countries, the EPA suggests that the UV lamps be maintained regularly and follow one of three cleaning techniques. There is "off-line chemical cleaning," which requires the tank to be drained and the lamp to be cleaned with chemicals. "On-line mechanical cleaning" consists of wipers that mechanically clean the lamp without needing to drain the reactor tank. "On-line-mechanical-chemical cleaning" involves mechanically cleaning the lamp with wipers and concurrently using chemicals to clean the lamp.[5] The lamp sleeves should be replaced periodically and aging of the lamp should be monitored, replacing the lamp when its efficiency decreases to 70%.[11]
Evaluation of Disinfection System[edit | edit source]
Technical Evaluation[edit | edit source]
Effluent water quality testing is one of the best indicators of how well the disinfection system is performing. Testing water quality in a developing area is made possible by using Petrifilms from 3M and plastic pipettes. To test, a sample of the effluent must be placed on the Petrifilm, and incubated for the designated number of hours. Body heat is often used as an incubator due to the lack of lab-grade incubators found in the field. The sample may need to be diluted if the E-coli or total coliform count on the Petrifilms is greater than 150.[23]
Turbidity is also a pertinent test to determine effectiveness of the treatment system. Turbidity can be measured using either a turbidimeter, a transparency tube,[24] or a secchi disk.[25] To measure turbidity using a secchi disk, the disk is lowered into the water and the depth at which the disk is no longer visible is recorded for a turbidity estimation.[25] This method works best when a large volume of water must be tested, but can be adapted to a smaller volume.
Social Evaluation[edit | edit source]
Many water treatment technologies fail due to the lack of acceptance within the community. A good measure of success for any technology within a community would be how many people are using the technology, and how many people report that they are satisfied with the new technology. For water treatment technologies specifically, an important indicator of success is rate of sickness when using the treatment system versus rate of sickness when not using the system. If the user is not properly using the technology, the treatment effectiveness becomes null.
Advantages and Limitations of UV Disinfection[edit | edit source]
Advantages[edit | edit source]
- An inexpensive alternative to many other disinfection methods.[6]
- UV disinfection is 20000 times less energy consuming than boiling water to disinfect it, which is the current method for many people.[10]
- Simple to operate and maintain[10]
- Relatively fast disinfection - short reactor retention time[10]
- No change in taste[10]
- No residual odor from disinfection[10]
- Has no toxic byproducts like chemical disinfectants and ozone[6]
- Simple installation[11]
- Little space requirement[11]
- UV-C inactivates all known bacteria and most known viruses[26]
- pH and temperature do not affect UV disinfection process[11]
Limitations[edit | edit source]
- UV lamps and quartz casing may not be locally available
- UV lamps must run with continuous electricity
- UV lamps contain mercury, which is toxic and difficult to dispose of[4]
- Bacteria can repair themselves if UV dose is not high enough[5]
- There can be byproducts from UV disinfection that result from reactions between phytochemicals[5]
- UV-C cannot effectively inactivate Adenoviruses[26]
- Poor disinfection when influent has high levels of suspended solids, turbidity, color, or organic matter[11]
External links[edit | edit source]
Wikipedia articles: Wikipedia:Ultraviolet germicidal irradiation
Wikipedia:Plug flow reactor model
References[edit | edit source]
- ↑ WHO | World Health Organization. (2010). Retrieved June 8, 2010, from http://www.who.int/en/
- ↑ Murinda, S., & Kraemer, S. (2008). The potential of solar water disinfection as a household water treatment method in peri-urban Zimbabwe. Physics and Chemistry of the Earth, Parts A/B/C, 33, 829-832.
- ↑ Gadgil, A. J., & Derby, E. A. (2003). Providing Safe Drinking Water to 1.1 Billion Unserved People. Presented at the AWMA Conference, San Diego, CA.
- ↑ 4.0 4.1 4.2 Chatterley, C., & Linden, K. (2010). Demonstration and evaluation of germicidal UV-LEDs for point-of-use water disinfection. Journal of Water and Health, 8.3.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 Schmelling, D., Cotton, C., Owen, D., Mackey, E., Wright, H., Linden, K., & Malley, J. U.S. Environmental Protection Agency, Office of Water. (2006).Ultraviolet disinfection guidance manual for the final long term 2 enhanced surface water treatment rule. Retrieved from U.S. Government Printing website: http://web.archive.org/web/20130506062427/http://www.epa.gov/ogwdw/disinfection/lt2/pdfs/guide_lt2_uvguidance.pdf
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Acher, A., Fischer, E., Turnheim, R., et al. (1997). Ecologically friendly wastewater disinfection techniqes. Water Research, 31:6, 1398-1404.
- ↑ Mihelcic, J. R., Fry, L. M., Myre, E. A., Phillips, L. D., & Barkdoll, B. D. (2009). Field guide to environmental engineering for development workers: Water, sanitation, and indoor air. Reston, VA: American Society of Civil Engineers.
- ↑ Garrison, L.M., Murray, L.E., Doda, D.D., and Green, A.E.S. (1978). Diffuse-direct ultraviolet with a compact double monochromator. Appl. Optics, 17, 827-836.
- ↑ Gadgil, A. (1998). Drinking water in developing countries. Annual Reviews of Energy and Environment, 23:253-86.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 10.12 Gadgil, A. (2008). Safe and affordable drinking water for developing countries. Presented at the AIP Conference Proceedings, Berkeley, CA.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Tech brief: Ultraviolet disinfection. (2000, September).National Drinking Water Clearinghouse, 15, Retrieved from http://web.archive.org/web/20191231005320/http://www.nesc.wvu.edu/ndwc/pdf/ot/tb/ot_tb_f00.pdf
- ↑ 12.0 12.1 Benjamin, M., & Lawler, D. (2009). Water quality engineering: Physical and chemical treatment processes. New York, NY: The McGraw-Hill Companies.
- ↑ Rittmann, B. E., & McCarty, P. L. (2001). Environmental biotechnology: Principles and applications. (1 ed., pp. 262-264). New York, NY: McGraw-Hill Book Co.
- ↑ 14.0 14.1 14.2 14.3 Gadgil, A. (1996). UV Waterworks: Reliable, Inexpensive Water Disinfection for the World UVW: UV Waterworks. Retrieved June 6, 2010, from http://web.archive.org/web/20170208151116/https://eetd.lbl.gov/newsletter/cbs_nl/NL09/cbs-nl9-waterworks.html
- ↑ Gadgil, A., Drescher, A., Greene, D., Miller, P., Motau, C., Stevens, F. (1997) Field-testing UV Disinfection of Drinking Water. Presented at the WEDC conference “Water and Sanitation for All,” Surbon, South Africa.
- ↑ 16.0 16.1 U.S. Department of Energy, Office of Science: Lawrence Berkeley National Laboratory. (n.d.). Success stories: Waterhealth international. Retrieved from website: http://www.lbl.gov/tt/success_stories/articles/WHI_more_no_Mex.html
- ↑ Gadgil, A. (1998). Drinking water in developing countries. Annual Reviews of Energy and Environment, 23:253-86.
- ↑ Uv tube. (2006). Informally published manuscript, Renewable and Appropriate Energy Laboratory, University of California - Berkeley, Berkeley, CA. Retrieved from http://uvtube.berkeley.edu/home
- ↑ Niparaja, A. C. (2009). Highlighted invennovations: Uveta. Retrieved from http://www.invennovations.com/waterpurification.html
- ↑ 20.0 20.1 20.2 Barstow, C. K. (2010). Development of an ultraviolet point-of-use device for household water disinfection. (Master's thesis, University of Colorado at Boulder)Retrieved from https://ceaemgmt.colorado.edu/ceae/images/File/mcedc/Thesis Barstow_Final.pdf
- ↑ Uv tube. (2006). Informally published manuscript, Renewable and Appropriate Energy Laboratory, University of California - Berkeley, Berkeley, CA. Retrieved from http://uvtube.berkeley.edu/home
- ↑ Linden, K., et al. (2009). Demonstrating 4-log adenovirus inactivation in a medium-pressure UV disinfection reactor. Journal AWWA, 101:3.
- ↑ 3M. (2001). Petrifilm e.coli/coliform count plate: Interpretation guide. Retrieved from http://web.archive.org/web/20130212082726/http://www.3m.com/intl/kr/microbiology/p_ecoli/use3.pdf
- ↑ Myre, E., & Shaw, R. (2006). The turbidity tube: Simple and accurate measurement of turbidity in the field. (Master's thesis, Michigan Technological University)Retrieved from http://www.cee.mtu.edu/sustainable_engineering/resources/technical/Turbidity-Myre_Shaw.pdf
- ↑ 25.0 25.1 Lenntech. (2011). Turbidity. Retrieved from http://www.lenntech.com/turbidity.htm
- ↑ 26.0 26.1 Gerba, C.P., Gramos, D.M., & Nwachuku, N. (2002). Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Applied & Environmental Microbiology., 68:10:5167.
Aquafine Corporation. (2011). Uv technology: The science of ultraviolet light. Retrieved from http://www.aquafineuv.com/UVTechnology/UVScience.aspx
Mounaouer, B., Hamed, B. N., Helmi, H., et al. (2010). Modeling of secondary treated wastewater disinfection by UV irradiation: Effects of suspended solids content. Journal of Environmental Sciences-China, 22:8, 1218-1224.
Norma, A. B., Blanca, E. J. (2009). UV light effect on fecal coliforms, fecal streptococci, Salmonella typhi and Acanthamoeba spp. Ingenieria Hidraulica en Mexico, 24:3, 23-24.