This is a speculative class project and needs to be refined before being deployed at any scale.
This project is centered around the recycling process of the semiconductors in LED televisions, and how it could be improved upon. The brand of televisions focused upon is produced by LG Electronics of South Korea. Light emitting diodes (LEDs) are a semiconductor based lighting device that are quickly becoming the industry standard for many applications. One of the most popular applications is backlighting for liquid crystal display (LCD) panels.[2] LED backlighting is quickly becoming the industry standard for LCD screens of any size. Previously, most LCD screens (other than those used in handheld devices) were backlit using Cold Cathode Fluorescent Lamps (CCFLs). The switch to LED backlighting was prompted by market demands and the advantages of LED lighting. Compared to CCFLs, LEDs offer a lighter, thinner and more energy efficient display while better picture quality and a longer product life.[3] LEDs used for backlighting in LCD screens estimated lifetimes up to 50,000 hours (over 5 years of continous use). If an LED fails, the screen will produce uneven lighting or distorted colors.[3]Given the long lifetime of the LEDs used, it is likely that most televisions to be recycled would have failed through other means, such as mechanical damage, a power surge or obselecense.
Semiconductors Used
White light LEDs are the most commonly used LEDs for backlighting applications. RGB arrays, consisting of a red, green and blue LED are also used, but the colors degrade at different rates, leading to color misbalance in the picture. The most common method of producing white light from an LED is using a blue LED coated with specific materials that convert blue light to a wider spectrum centered around yellow.[4] Typically, the blue LED is a indium gallium nitride (InGaN) or gallium nitride (GaN) based semiconductor with a coating yttrium aluminum garnet doped with cerium. While exact procedures for manufacturing these semiconductors are trade secrets, the most common procedure appears to be metalorganic chemical vapor deposition (MOCVD), in which the precursors are reacted in the form of ultra-pure gasses. The gasses react with the substrate, and the specific atom is deposited on the surface.[5]

InGaN is a mixture of gallium nitride with small amounts of indium nitride to control the bandgap. This, in turn, controls the wavelengths of light emitted. Both InGaN and GaN are direct bandgap semiconductors, meaning that spontaneous emission of a photon does not involve phonon emission or absorption. This, in turn, allows for quicker emission of light and more efficiently operating LEDs. GaN has been used for violet LEDs since the 1990s, and is commonly used in applications such as Blu-ray Disc readers.
The first step of producing InGaN or GaN semiconductor devices is the substrate. Silicon carbide and sapphire substrates are both used. The substrate heated in a chamber with an atmosphere of a metalorganic gas that contains the desired atom to be deposited. The gas molecules are either broken apart by the heat of the chamber, or react with the substrate to deposit a thin film of atoms. The gas composition is changed to alter the layers deposited.[7] By altering layer thickness and composition, complex semiconductor devices can be produced.
LED TVs in the Market[edit | edit source]
LG revealed that they were forecasting their sales to increase from 3.1 million units, which was around 3% of the LED-backlit LCD TVs in the market, in 2009 to around 30 million units in 2010, which would be approximately 20% of all the TVs in the market. They were also predicting that the market would increase to 68 million units by 2011, becoming 40% of the market .[8]
Semiconductors in the Market
LG claimed that there were 3,360 LEDs in each unit (specifically the 55" screen TV) that they planed to sell in 2009. They sold over 3 million units that year. Which makes the total number of LEDs sold over 10 billion in 2009.[8] That is just for LG alone. Samsung and other electronic companies also had many LED devices sold.
Current Recycling Processes[edit | edit source]
Contaminants
LEDs, compared to previously used light sources, are very environmentally friendly. Their lifespan is much larger than the conventional incandescent bulbs, uses less electricity, and uses much cleaner technology than most. However like most technologies today, there are some forms of toxic contaminants. The three main contaminants in most LED bulbs are lead (Pb), copper (Cu) and nickel (Ni). Now in small amounts these metals are not lethal but when combined with millions of other LEDs, can be a hazardous issue. When recycling these bulbs, such contaminant issues must be kept in mind.
Recycling Procedure
Right now the most common procedure for recycling LEDs is the destruction and then collection of each individual components. The crushing of the LED is done by a bar screen which allows for the separating of the device into components. The crushed LED is then passed through a magnetic field that will remove any ferrous metal, leaving the non ferrous metal and glass by itself. A non ferrous metal separator is then used to separate metals such as aluminum and lead out of the glass. Once this is done the glass and metals can be purified and reused again.
Purification Methods
Purification and analysis of purity in indium and gallium
The extraction process outlined earlier in this report produces indium and gallium with approximately 99% purity. These metals must be further purified before they are used in the semiconductor manufacture process.
Indium can be futher purified by electrorefining from a sulfuric acid solution. The indium that was obtained from the previous extraction steps is reacted with sulfuric acid to form In2(SO4)3 in a solution of sulfuric acid. A current is applied to this solution and solid indium forms on one of the electrodes. This procedure has been shown to produce purities up to 99.9999% pure indium.[9]
Gallium can also be electrorefined, although solvent extraction appears to be more popular. Several methods are outlined in here
To determine the purity of the metals produced, a combination of optical spectroscopy techniques (atomic absorption/atomic emission/flame emission), along with mass spectroscopy could be used. It should be noted that these metals will be reacted again before they are used to produce LEDs via MOCVD processes. During this reaction process, they are likely selectively reacted or further purified. In general, the raw supply for indium and gallium are both low purity (<99%) when produced from mining companies. The low purity product could be sold directly to the same customers to reduce the processing done in the plant.
Other Recycling Processes
The process of recycling stated above is all under the category of straight recycling. This means that current, used semiconductor materials are recycled and then enhanced with new materials to enhance its properties to like-new qualities. Another form of recycling is down cycling. This is the process where you take recycled material from useless products and then recycle them into other products of lesser value. Down cycling would definitely be a much cheaper process. It would allow the recycler to produce a semiconductor that is less pure. This would also allow for the semiconductor to have cheaper and more available dopants.[10] On the other hand the use of down cycling would produce much more inferior material. This could cause more issues in the quality and life of the semiconductor.
Collection of semiconductor materials[edit | edit source]
By the year 2013 the market is expected to exceed 100 million units sold. In a recent study it is believed that over 95% of a semiconductor material in LED's can be recycled. The average LG TV has around 1500 bulbs in the screen (number changes if size of TV changes) and each LED weighs around 0.000227 kg. This means that around 34,050,000 kg of LED will be produced and 32,347,500 kg of that number can be effectively recycled.[11] Right now there are several drop off places around the world for your LG LED TV's but if you wanted to you could take apart your TV and remove the LED's and take them to individual stores such as Radio Shack or Home Depot and have them recycle the LED. [verification needed] The recycling process that we would put into effect is a way of either dropping off used LED TV's at designated drop off points and/or setting up some sort of alliance with LG. This alliance would allow us to take the used LED from the recycled TV's, recycle them and then sell them back to LG for less cost then it would take to make them. Right now the largest issue in recycling LED TV's and other electronics is to get the general public on the same page. A study done in 2009 showed that only an average of 13%-18% of all electronics are recycled. [verification needed] This means that most of electronics produced are put into a landfill and allowed to go to waste. With the growth of electronics increasing exponentially everyday, the need for a proper recycling of LED's and all electronics is very important as well as public support and backing.
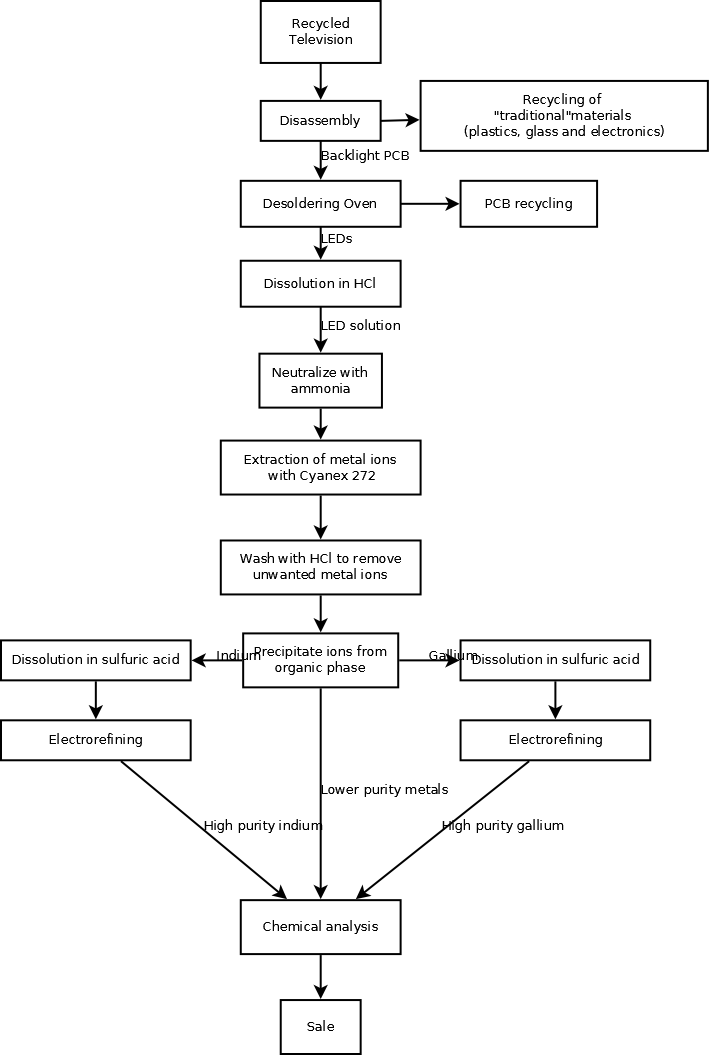
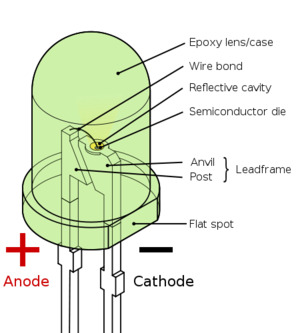
The challenge that arises from recycling of LEDs is the tiny size of each LED package, along with the complexity of each module. The semiconductor part of the LED is encased in an epoxy, surrounded by a case with metallic contacts. The first step of collecting the semiconductor materials would be to disassemble the television, performed by workers with some training. The LED backlighting module would be separated from the rest of the television. From here, an oven could be used to de-solder the LEDs from the PCB. The LEDs would be soaked in a hydrochloric acid solution for approximately 24 hours to be fully dissolved. The metal ions would be extracted from the aqueous solution by neutralizing the acid with a dilute base (such as ammonia), then reacting with bis(2,4,4-trimethylpentyl)phosphinic acid (commercial name Cyanex 272). Further cleaning with additional HCl washes would remove the metal ions from the contacts.[12]
Recycling, Collection and Transportation[edit | edit source]
Recycling
During the process of recycling, there are many cost and energy requirements that are needed for the purification of the LED semiconductor.
Chemicals:
- ) HCl ~ $300/ton
- ) Ammonia ~ $500/ton
- ) Cyanex 272 ~ Cost/ton Unknown
- ) Sulfuric Acid ~ $250/ton
Disassembly of TV's:
depends on the tools and labor used
Desoldering Oven:
Cost ~ $1000-$2000/unit (depending on size of unit) Energy Usage ~ 2500 watts
Collection and Transportation
Ideally the collection facility and recycling plant would be relatively close. In the US most recycling facilities are not close but all across the country. When the facility is close the cost of transportation is very low.
Plant Design[edit | edit source]
Facility Specifications[edit | edit source]
The facility that would be used to disassemble LED televisions and then recycle the components of each LED needs to be able to handle a minimum of 20 million units at a given time. This is about 20% of the projected number of units in the market for next year. Because the usage of LEDs continues to grow substantially, the plant must be able to expand in order to keep up with the production of LEDs.
LED removal[edit | edit source]
The majority of the work done in the plant would be done by individual workers stationed at work benches. Each worker would require a set of tools for disassembly of the televisions, including screwdrivers (in a variety of sizes and bits), pry bars, pliers, etc. The amount of workers and work stations required would depend on the throughput of TVs to be recycled. It would likely take an experienced worker approximately 20 minutes to disassemble a television.
To desolder the LEDs from the PCB, two methods are available. First, workers could use a hot air gun to melt the solder and individually pick the LEDs off of the board. This would be time consuming, but would save on the initial costs. The second option is put the PCBs in a large oven at low temperatures. The PCB would be vibrated gently assist the LEDs falling off of the board. Only one oven would be needed due to the short time needed to desolder electronic components.
Semiconductor Material Flow Diagram[edit | edit source]
Extraction and purification[edit | edit source]
Due to the relatively small size of LEDs used in backlighting applications, the extraction and purification processes would likely take place in a laboratory type environment. A small (approximately 10 litres or less) reactor vessel would be necessary for the initial dissolution of the LEDs in hydrochloric acid. Five more reactor vessels of similar size would necessary for the extraction, washing and precipitation steps. In addition, an additional 1-2 reactors might be necessary due to the long dissolution time of the first extraction step.
For electrolytic purification, two electrolytic cells would be necessary (one for indium and one for gallium). Each cell would be small compared to industrial electrorefining equipment in the primary metals industry. The electrodes would be approximately 10cm x 10cm square. Power supplies would be necessary to run each of the cells.
To determine the purity of the refined metals, specialized testing equipment would be necessary. Depending on the needs of the customers, a spectrometer would be chosen to analyze each batch of product. A likely tool to be used is inductively charged plasma emission spectroscopy, which would likely match the purity of the product produced.
Safety Procedure[edit | edit source]
MSDS for Chemicals Used
HCl: http://web.archive.org/web/20181215084837/http://www.sciencelab.com:80/msds.php?msdsId=9924285
Ammonia: http://www.agriumwholesale.com/includes/msds/AA820000ReferMSDS.pdf
Sulfuric Acid: http://web.archive.org/web/20120131151110/http://www.ee.iitb.ac.in:80/~nanoe/msds/sulphuric%20acid.pdf
Safety Plan
The safety plan will be put in place to allow for the protection and well being of all workers involved. The plan will focus on the most important aspects below:
- MSDS sheets and how to use them
- Proper handling techniques of materials and chemicals
- How to clean up spills and messes in the facility
- How to use and execute safety protocols in case of an emergency
- The usage of proper lab safety equipment
References[edit | edit source]
- ↑ http://en.wikipedia.org/wiki/Light-emitting diode
- ↑ http://en.wikipedia.org/wiki/Light-emitting_diode
- ↑ 3.0 3.1 Moon-Hwan Chang, Diganta Das, P.V. Varde, Michael Pecht, Light emitting diodes reliability review, Microelectronics Reliability, Volume 52, Issue 5, May 2012, Pages 762-782, ISSN 0026-2714, 10.1016/j.microrel.2011.07.063. (http://www.sciencedirect.com/science/article/pii/S0026271411003283)
- ↑ A nearly ideal phosphor-converted white light-emitting diode Steven C. Allen and Andrew J. Steckl, Appl. Phys. Lett. 92, 143309 (2008), DOI:10.1063/1.2901378
- ↑ US patent 7186302, Chakraborty; Arpan, et al., "Fabrication of nonpolar indium gallium nitride thin films, heterostructures and devices by metalorganic chemical vapor deposition", issued 2007-03-06, assigned to The Regents of the University of California & The Agency of Industrial Science and Technology
- ↑ https://www.appropedia.org/File:MOCVDprocess.jpg
- ↑ US patent 4736705, Weyburne; David W., "Apparatus for metal organic chemical vapor deposition", issued April 12, 1988, assigned to The United States of America as represented by the Secretary of the Air
- ↑ 8.0 8.1 http://ledsmagazine.com/features/6/8/6
- ↑ http://www.springerlink.com/content/f868244012136un6/
- ↑ http://en.wikipedia.org/wiki/Downcycling
- ↑ http://www.ledinside.com/knowledge/2012/7/what_is_led
- ↑ Bina Gupta, Niti Mudhar, Indu Singh, Separations and recovery of indium and gallium using bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272), Separation and Purification Technology, Volume 57, Issue 2, 15 October 2007, Pages 294-303, ISSN 1383-5866, 10.1016/j.seppur.2007.04.011. (http://www.sciencedirect.com/science/article/pii/S1383586607001876)