This is a speculative class project and needs to be refined before being deployed at any scale.
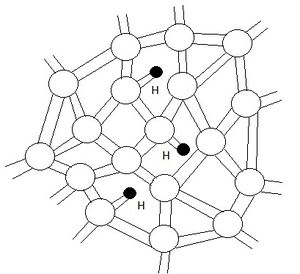
The technology that me and my colleagues have chosen to examine as a possible semiconductor recycling project is the LCD PC monitor. We have chosen Dell[1] to take a closer look at the viability of recycling semiconductors. Hydrogenated amorphous silicon is the typical choice of semiconductor material in the manufacturing of LCD monitors. It is the best choice of semiconductor material because it can be "grown" over large areas. The structure of this silicon is not that of a crystalline material. It lacks the long range order that is present in crystalline silicon. Within the material there are vacancies that form, leaving behind dangling bonds; these free bonds are free electrons from the silicon that have not bonded with another silicon atom. However, the addition of the hydrogen atoms reduces the amount of dangling bonds because hydrogen consists of one free electron and can attach to the vacant bonds. This can be seen in Figure 1.[2]
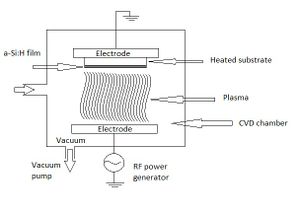
The process of which the hydrogenated amorphous silicon is deposited onto a substrate is called plasma-enhanced chemical vapor deposition (PECVD). This process uses silane gas which enters the chemical vapor deposition chamber and dissociates into a cloud of plasma. This plasma is induced by a radio frequency power generator; the chamber that holds this process must be in a vacuum to eliminate any impurities in the process. Similiar to an electron beam deposition process,the silicon and hydrogen atoms condense on a substrate, in LCD monitors this would be the glass, and make the thin layer of hydrogenated amorphous silicon. The setup of this process is shown in Figure 2.[3]
Scale of market[edit | edit source]
The production of PC LCD monitors on the worldwide market shows Dell, Samsung, and LGE at the top of the market selling most for the 12 Brands selling from as low as 10 million units in a month to nearly 15 million units a month for 2010. Dell was ranked number one in the production of LCD for 2010. We were capable of determining the amount of units sold by Dell, which is estimated at 22.5 million units in 2010.[4]
In the total amount of PC LCD monitors that the top 12 brands sold between January 10 2009 and January 10 2010 was approximately 139 million units.[5]
The growth for the production of LCD screens is expected to slowdown in the next two years. The growth in production of LCD panels for LCD has decreased, by the slow economic recovery, which is causing consumers to buy less and is predicted to slow down further for the next few years.[6]
Recycling practices[edit | edit source]
Yes, LCD monitors are recycled.[edit | edit source]
Scrap LCD monitors are sold for recycling of the actual LCD panel when its not cracked. If it is cracked then there is no longer a value.[7]
How to recycle your LCD monitors…[edit | edit source]
To prevent your old electronics from being melted down using unsafe practices or to be tossed in a land fill, then recycling is your calling.[8]
Recycling LCD monitors is easy if you do not have to go through the process yourself. There are multiple companies that allow you to send in your monitors for free, for a price or you can even drop them off at specific locations and they will handle it all for you. Some of these companies include Apple, Staples and Dell. Michigan Technological University allows people to drop off their monitors and the Apple store will recycle it all. If you want to go straight through Apple, their recycling process is to purchase any Apple computer or monitor and receive free recycling of your old computer and monitor no matter what the brand is.
Why it should be recycled…[edit | edit source]
Computers consist of valuable resources, such as precious metals, copper, and engineered plastics. Recycling computers enables us to collect and reuse these valuable resources. For example, by recycling 100 million cell phones, about 7,500 pounds of gold could be recovered. Recovering this gold, instead of mining it, would prevent 12,000,000,000 pounds of loose soil, sand, and rock from having to be moved, mined, and processed.[9]
Dangers of not recycling properly...[edit | edit source]
Electronic waste isn't just waste. It contains contaminants, such as mercury, lead, cadmium, arsenic, and beryllium. When these contaminants are burned at low temperatures they create more toxins and are released into the air. The toxic materials in electronics can cause cancer, reproductive disorders, endocrine failures and many other health problems if the waste is not properly disposed of. An estimated 70-80% of the electronic waste is exported to third-world countries. Once there the toxins are released into water sources and the land.[10]
What can the recycled be used for…[edit | edit source]
Scientists have found that by separating the panels in LCD screens, they can remove the polyvinyl-alcohol (PVA) and then produce a disinfectant. This substance is able to kill harmful bacteria including E-coli.
Andrew Hunt of the University of York says, "We can add significant value to this waste...that has great potential for use in biomedicine. Now we have gone a step further by enhancing its anti-microbial properties through the addition of silver nanoparticles, with the result being that it can destroy bacterial infections."[11]
Amount of semiconductor in market[edit | edit source]
The amount of hydrogenated amorphous silicon is all dependable on the thickness of the thin film layer. These layers can range from nanometers to micrometers, and for the LCD monitors an average between the two will be used to determine the thickness.[12]
To determine the total volume in each monitor that Dell sells, we took the average height and width of the monitors that are offered.[13]
Converted to meters, the average size of the screen is:.592x.364 meters
To determine the mass of the silicon present, we took the average volume of the monitors and multiplied it by the density of silicon.
Finally, the total amount of silicon that could be possibly extracted from the 22.5 million units sold is:
5,654,250=12,465.487 lbs
Methods of collecting lost semiconductor materials[edit | edit source]
The methods for collecting the liquid crystals from the LCD panels are:
- Supercritical Carbon Dioxide Fluid Technology extracts the liquid crystals from the glass. This method uses iso-thermal and a depressurization method to remove the liquid crystal from the glass panel, by converting carbon dioxide gas to its supercritical fluid state, thus dissolving the liquid crystal. Then the temperature is dropped, the carbon dioxide reverts back to gas, leaving the liquid crystal[14].
- Ultrasonic cleaning uses ultrasonic waves, which causes pressure against the liquid, thus removing the liquid crystals by force from the glass substrate[15].
After the liquid crystals have been extracted and removed and cleaned using a solvent, they can be recycled back into a different LCD[16][17].
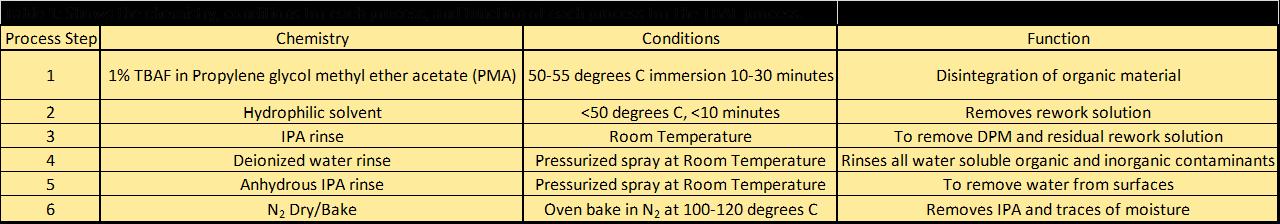
Stripping Process[edit | edit source]
This process involves the use of a base, usually quaternary ammonium hydroxide, a surfactant and a high boiling solvent (di- or tri- propylene glycol alkyl ether.) to strip the silicon.[18]
Mechanism of Cured Silicone Adhesive Removal with TBAF[edit | edit source]
The cured silicon can be exposed to tetrabutyammonium fluoride reagent (TBAF) in non-hydroxylic aprotic solvent. This causes a disintegration of the polymer matrix, thus removing the silicon into the solvent.[19]
This process is extremely viable for removing the semiconductor silicon adhesive off of the TFT or many other types of substrates.
Potential for Post-Consumer Recycling[edit | edit source]
It has been decided that the semiconductor material found in Dell LCD PC monitors is a viable resource that should be recycled post-consumer.
Part A[edit | edit source]
Collection Methods[edit | edit source]
Recovered
Wasted
Percent Recovered:
Contaminants[edit | edit source]
Mercury is the contaminant found in the monitors. Some computer monitors that use liquid crystal display (LCD) technology contaisn mercury, a highly toxic metal that can cause serious damage if ingested. The mercury in monitors is there to produce light when it is electrically energized. When the laptop monitors are tilted, mercury flows to one end cutting off the circuit and opening it on the opposite end. This is often function of an on and off switch.[20]
At Stena Innovative Recycling, they clean units by separating it into iron, metals, plastics, circuit boards and glass with liquid crystals. The whole process works in a closed and controlled environment and during the process the levels of mercury are controlled, so Stena can be sure that all the mercury is removed from the material that will be recycled. The units that are contaminated with mercury are then sent to a hazardous waste treatment center.[21]
The most efficient waste treatment process is to separate the backlight lamps from the panel. If the lamp is not taken out before, the whole display is deemed hazardous waste. Many treatment processes were explored, such as water-jet cutting, laser cutting and circular sawing, but the most efficient way is by manual dismantling. Costs per unit and the assessment quality were variables in the processes explored.[22]
The concentration of Mercury found in each unit is about 2mg.
1663.60 Kg of Mercury sold from LCD monitors in 2000
Purification Methods[edit | edit source]
The purity of silane is 99.9999 percent. Electronically active impurities, such as boron, phosphorus, and arsenic are controlled to less than 10 parts per trillion. Silane is one of the purest materials on Earth.[23]
The manufacturing process of silane that is used by REC produces consistent, pure silane by converting metallurgical grade silicon into trichlorosilane and redistributiing and distilling to silane. The constant flow process, recycles all hydrogen and chloride to initial reactors, while constant distillation steps purify the gas. This process is environmentally friendly.[24]
In the most simple way to describe the production of silane, silicon is turned into a gas by grinding it down to a fine, sand-like consistency and heating it with hydrogen and silicon tetrachloride. After this is done it is then put through a series of reactions, as seen below, and silane and pure polysilicon are made.
Industrially, silane is produced from silicon in a two-step process. In the first step, powdered silicon reacts with hydrogen chloride at 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas.
Si + 3 HCl → HSiCl3 + H2
The trichlorosilane is then boiled on a resinous bed containing a catalyst which allows the formation of silane and silicon tetrachloride.
4 HSiCl3 → SiH4 + 3 SiCl4
Another way to process silane is to start with metallurgical grade silicon, hydrogen, and silicon tetrachloride and let them go through a series of redistribution reactions and distillations as seen below:
Si + 2 H2 + 3 SiCl4 → 4 SiHCl3
2 SiHCl3 → SiH2Cl2 + SiCl4
2 SiH2Cl2 → SiHCl3 + SiH3Cl
2 SiH3Cl → SiH4 + SiH2Cl2
The silane produced by this process can be thermally decomposed to produce high-purity silicon and hydrogen in a single pass. Another commercial production of silane involves reduction of SiO2 under Al and H2 gas in a mixture of NaCl and AlCl3 at high pressures:
3SiO2 + 6H2 + 2Al → 3SiH4 + Al2O3[25]
In-Situ Analysis[edit | edit source]
In-situ is defined as an object being situated in a place of its natural state or localized area.[26]
In comparison to our characterization methods to purify our material, the in-situ analysis can be adapted by looking at the comparison of what our purity level is compared to the purity level found in nature.
Cost For Recycling Compared to Manufacturing Semiconductors[edit | edit source]
The cost to transport to recycling center is estimated at 0.0172 dollars per kilogram.[27]. From our calculated total amount of world wide recovered amount of semiconductors is 43,885.29 Kg.
The cost to sort semiconductors is 0.140 dollars per Kg.
The cost to dismantle them is 0.315 dollars per Kg.
Cost to recycle semiconductor per kg is unknown at this current time.
As this figure is unknown, we can still determine what the cost per kg of recycling of the monitor must be less than in order to be a practical recycling process.
The total cost to produce the yearly amount of 3000,000 kg[28] of the semiconductor silane is $4,202,331.00.
So we get the total cost of producing silane per kg by taking
The total cost to recycle the silane must be lower than the cost to manufacture minus the cost to collect and transport the silane, thus
which needs to be greater than the cost to recycle the silane.
It must cost less 13.5356 dollars per kg to recycle silane[29].
Alternative Recycling[edit | edit source]
Alternatively instead of straight recycling only the silane recovered from the LCD PC monitors back into new monitors, we can also collect and extract the silane from other electronic devices that contain silane. The silane can also be put in other electronics devices with LCD screens. The silane can be used in the production solar panels, LCD TVs, smartphones, and other electronic devices which either have a LCD screen or need the silane to connect the glass to the polymer matrix in these devices.[30]
Down cycling[edit | edit source]
One of the alternatives to straight cycling of the Silane is down-cycling it into titanium implants, so the biologically inert material in the implant can attach to the titanium implant. The recovered Silane can also be used as water repellent and masonry protection. The Silane can also be used for initiating the combustion for ramjets, reaction engines and liquid fuel rockets that have carbon dioxide in it.[31]
Pros & Cons[edit | edit source]
As with any form of alternative recycling such as downcycling there are goods things and there are bad things. The good thing about downcycling is the silane is reused instead of extracting the silane from the Earth and having to go through the process of purification, which all require labor, energy, and other expenses. The bad thing about downcycling the silane is the loss in value of the silane. When the high quality silane extracted from the LCD PC monitor is downcycled into water repellent, then there is a tremendous loss in value of the silane.
Recycling Facility[edit | edit source]
Capital Equipment for Recycling Semiconductor grade Silicon[edit | edit source]
- Amerimade designs a quartz bath specifically for use with semiconductor materials. It can also be heated to desirable tempertures. It is a great machine for the first step.
- This bath is made from quartz and can be heated to desirable tempertures. This particular bath was designed specifically for use with semiconductor materials. It is the same piece of equipment as step one, however it is a great fit for step two as well.
- Jinan Bakr Ultrasonic Technology Co., Ltd. makes an industrial cleaner that runs on electric power and can be heated, although it is not necessary for this particular step. It is a great machine to use for the isopropyl alcohol rinse because it's also an ultrasonic cleaner.
- This industrial cleaner runs on electric power and can be heated, although it is not necessary for this particular step. It is a great machine to use for the deionized water rinse because it's also an ultrasonic cleaner.
- This industrial cleaner runs on electric power and can be heated, although it is not necessary for this particular step. It is a great machine to use for the anhydrous IPA rinse because it's also an ultrasonic cleaner.
- Gulf Coast Environmental makes industrial ovens engineered to your specific needs. These ovens will dry the substrate of any unwanted remaining chemicals so it will be able to be used in other applications.
Safety Plan[edit | edit source]
Eye protection must be worn at all times throughout the plant, ear protection is encourage, closed toe boots are recommended, and proper clothing made of non-synthetic material should always be worn. Over-head showers and eye washing stations will be found near the exits as well as the middle of most work stations. Fire extinguishers will be within 20 feet of every machine. Emergency exits will be thoroughly outlined on the maps as you enter each room. Although the facility is ventilated, there will be fume heads located toward the more hazardous chemicals will be dealt with as well as emergency fans nearest the windows. Hazardous waste disposal bins will be located near fume hoods. OSHA regulations will be strictly reinforced.
Silane is flammable and will ignite on contact with air. It is irritating to the eyes and skin. In case of emergency and silane comes into contact with the skin, wash the affected area with soap and water. In case of contact with eyes, flush with water for 15 minutes. In case of deep inhalation, seek medical attention immediately. When handling, avoid air contact and make sure that there is no source of ignition anywhere near. Electrical equipment needs to be explosion proof. Neoprene, butyl rubber or polyethylene gloves should be warn when handling. To store silane, keep away from bases such as halogens and other oxidizing agents. Disposal of silane must be done in a compressed gas distributor when no longer in use.[32]
MSDS Sheet Links[edit | edit source]
Silane http://web.archive.org/web/20140508200426/https://www.clean.cise.columbia.edu/msds/silane.pdf
Silicon http://dept.harpercollege.edu/chemistry/msds1/Silicon%20metal%20ScienceLab.pdf
Chloride http://web.archive.org/web/20181127014856/http://www.sciencelab.com:80/msds.php?msdsId=9927593
Hydrogen Chloride http://web.archive.org/web/20181215084837/http://www.sciencelab.com:80/msds.php?msdsId=9924285
Trichlorosilane http://alemis.us.airliquide.com/ChemSafe/MSDS/Image/115583_1.PDF
Silicon tetrachloride http://web.archive.org/web/20120507155851/http://www.airgas.com/documents/pdf/001075.pdf
Silicon Dioxide http://www.nanoamor.com/msds/msds_SiO2_7014WJQB.PDF
Sodium Chloride http://web.archive.org/web/20181003185825/http://www.sciencelab.com:80/msds.php?msdsId=9926835
Aluminum Chloride http://web.archive.org/web/20181208152806/http://www.sciencelab.com:80/msds.php?msdsId=9922851
Boron http://web.archive.org/web/20181003200317/http://www.sciencelab.com:80/msds.php?msdsId=9923126
Phosphorous http://web.archive.org/web/20150924114816/http://www.sciencelab.com/msds.php?msdsId=9927697
Arsenic http://web.archive.org/web/20181003202743/http://www.sciencelab.com:80/msds.php?msdsId=9922970
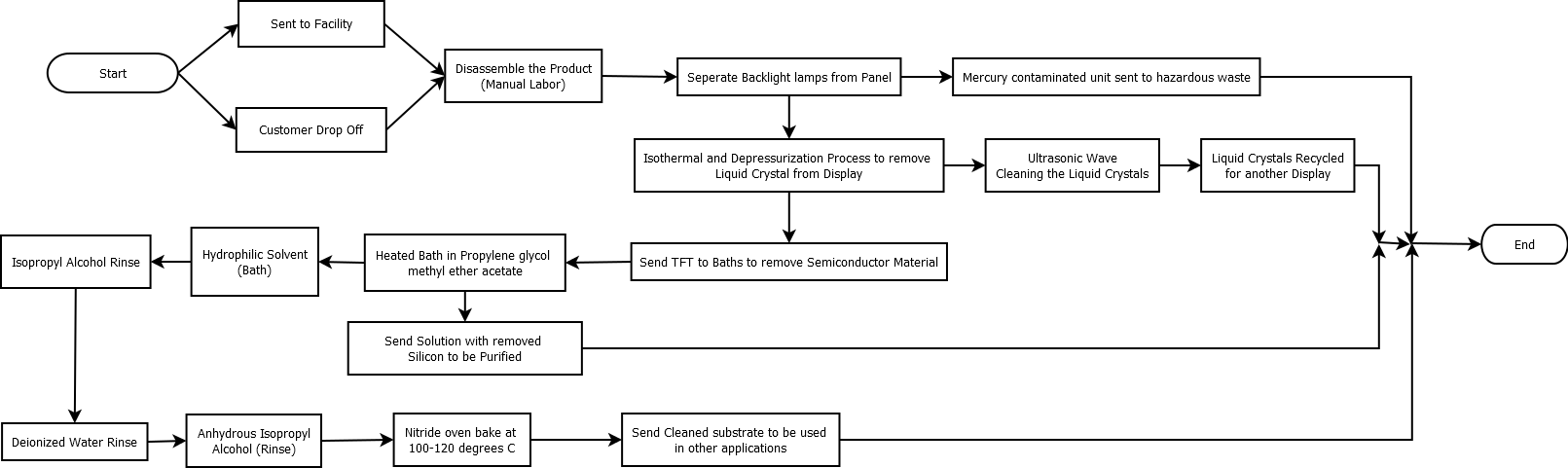
Material Flow Diagram[edit | edit source]
This is the optimal flow for the theoretical process of recycling the semiconductor and other materials in LCD PC Monitors. There are two ways of the recycling center receiving the monitors, either by customer drop off or shipment to the center. This process is optimal because it recycles all of the material in the monitors. Also, the process of removing the semiconductor from the substrate has been laboratory approved to efficiently and easily remove the material.
Resources[edit | edit source]
- ↑ "Dell Monitors.": Computer Monitor, LCD Display and Screen. N.p., n.d. Web. 25 Sept. 2012. <http://www.dell.com/monitors>
- ↑ Kasap, S. O. Principles of Electronic Materials and Devices. Boston: McGraw-Hill, 2006. Print.
- ↑ Kasap, S. O. Principles of Electronic Materials and Devices. Boston: McGraw-Hill, 2006. Print.
- ↑ "LCD Monitor Production Will Continue to Soar." TechEye. N.p., 07 June 2010. Web. 29 Sept. 2012. <http://news.techeye.net/business/lcd-monitor-production-will-continue-to-soar>
- ↑ "LCD Monthly Desktop Monitor Production Rate Highest Since Mid-2008 - DisplaySearch." LCD Monthly Desktop Monitor Production Rate Highest Since Mid-2008 - DisplaySearch. N.p., 01 Feb. 2010. Web. 29 Sept. 2012. <http://www.displaysearch.com/cps/rde/xchg/displaysearch/hs.xsl/100201_lcd_monthly_desktop_monitor_production_rate_highest_since_mid_2008.asp>
- ↑ Dash, Sweta. "Market Watch." LCD Panel Market Growth Slows in 2011. N.p., 19 May 2011. Web. 29 Sept. 2012. <http://www.isuppli.com/Display-Materials-and-Systems/MarketWatch/Pages/LCD-Panel-Market-Growth-Slows-in-2011.aspx>
- ↑ Grossman, Elizabeth. Salon. N.p., 10 Apr. 2006. Web. 16 Sept. 2012. <http://www.salon.com/2006/04/10/greenguide/>
- ↑ "LCD Monitors recycling." B.W Recycling Inc.. B.W. Recycling, Inc, 1 Sept. 2012. Web. 20 Sept. 2012. <http://www.computersrecyclingcompany.com/lcdmonitors.htm>
- ↑ "Reuse & recycle." U.S. Environmental Protection Agency, 16 Apr. 2012. Web. 18 Sept. 2012. <http://web.archive.org/web/20150823095008/http://www.epa.gov:80/epawaste/partnerships/plugin/reuse.htm>
- ↑ "Reuse & recycle." U.S. Environmental Protection Agency, 16 Apr. 2012. Web. 18 Sept. 2012. <http://web.archive.org/web/20150823095008/http://www.epa.gov:80/epawaste/partnerships/plugin/reuse.htm>
- ↑ "LCD toxic trash: useful antibiotic?." Smart Planet. Ed. Melissa Mahony. N.p., June 2010. Web. 7 Sept. 2012. <http://web.archive.org/web/20110517053646/http://www.smartplanet.com:80/blog/intelligent-energy/lcd-toxic-trash-useful-antibiotic/1663>
- ↑ <http://www.csun.edu/~rdconner/630/slides/Amorphous%20SC%20and%20Solar%20Cells.ppt>
- ↑ <http://www.dell.com/content/topics/segtopic.aspx/monitor_segselecter?c=us&cs=04&l=en&s=bsd&ST=dell%20monitors&dgc=ST&cid=245312&lid=4445170&acd=sTvOmguLW,26522075259,901w1k7137>
- ↑ "Study on Method Recycling Liquid Crystal from Waste LCD Based on Supercritical CO2 Fluid Technology" Scientific.net N.p., 27 Feb. 2012. Web. 29 Sept. 2012. <http://www.scientific.net/AMR.479-481.2165>
- ↑ "Recovery of Valuable Material from Waste Liquid Display Panel" Sciencedirect.com N.p., 7 Jul. 2009. Web. 29 Sept. 2012. <http://www.sciencedirect.com/science/article/pii/S0956053X08004315>
- ↑ "Recovery of Valuable Material from Waste Liquid Display Panel" Sciencedirect.com N.p., 7 Jul. 2009. Web. 29 Sept. 2012. <http://www.sciencedirect.com/science/article/pii/S0956053X08004315>
- ↑ "Recycling Liquid Crystal Displays (LCD)" voices.yahoo.com N.p., 11 Sept. 2006. Web. 29 Sept. 2012. <http://voices.yahoo.com/recycling-liquid-crystal-displays-lcd-78361.html>
- ↑ Sachdev, Krishna G. "Thus, Having Described the Invention, What Is Claimed Is:." REMOVAL OF CURED SILICONE ADHESIVE FOR REWORKING ELECTRONIC COMPONENTS. N.p., 03 Jan. 2002. Web. 12 Oct. 2012. <http://www.freepatentsonline.com/y2002/0000239.html>.
- ↑ "Removing Cured Silicone Adhesive from Electronic Components." - ElectroIQ. N.p., n.d. Web. 12 Oct. 2012. <http://web.archive.org/web/20120722020245/http://www.electroiq.com/articles/ap/print/volume-15/issue-10/features/removing-cured-silicone-adhesive-from-electronic-components.html>.
- ↑ Arvidson, Erik. "Recommended Management and Disposal Options for Mercury-Containing Products." Ehow. Demand Media, 7 Feb. 2012. Web. 2 Oct. 2012. <http://www.ehow.com/info_8750770_list-harmful-contaminants-computers.html#ixzz28KxPlkXq>.
- ↑ Falkenberg, Hedvig. "LCD RECYCLING." Stena Innovative Recycling. Stena, n.d. Web. 5 Oct. 2012. <http://stenatechnoworld.com/Monitor-glass-and-LCD-recycling/LCD/>.
- ↑ Kopacek,. "ReLCD: RECYCLING AND RE-USE OF LCD PANELS." N.p., 2008. Web. 5 Oct. 2012. <http://ewasteguide.info/files/Kopacek_2008a_WasteCon.pdf>.
- ↑ Jones, John. "Cleaning up Silicon." N.p., May 2011. Web. 12 Oct. 2012. <http://spinoff.nasa.gov/spinoff2000/ip8.htm>.
- ↑ REC. Renewable Energy Corporation ASA, n.d. Web. 12 Oct. 2012. <http://web.archive.org/web/20091226191552/http://www.recgroup.com:80/products/silane-gases/>
- ↑ Shriver and Atkins. Inorganic Chemistry (5th Edition). W. H. Freeman and Company, New York, 2010, pp 358.
- ↑ <http://web.archive.org/web/20150904061506/http://dictionary.reference.com:80/browse/in+situ>
- ↑ "Comparative Analysis of a Silane Cylinder Delivery System and a Bulk Silane Installation(ESH B001)" 31 Oct. 1995. Web. 12 Oct. 2012.<http://web.archive.org/web/20060812013527/http://www.sematech.org:80/docubase/document/2976aeng.pdf>
- ↑ "Silane" Wikipedia.org, 20 Sept 2011, 13 Oct. 2012, <http://en.wikipedia.org/wiki/Silane>
- ↑ "Comparative Analysis of a Silane Cylinder Delivery System and a Bulk Silane Installation(ESH B001)" 31 Oct. 1995. Web. 12 Oct. 2012.<http://web.archive.org/web/20060812013527/http://www.sematech.org:80/docubase/document/2976aeng.pdf>
- ↑ "Silane" Wikipedia.org, 20 Sept 2011, 13 Oct. 2012, <http://en.wikipedia.org/wiki/Silane>
- ↑ "Silane" Wikipedia.org, 20 Sept 2011, 13 Oct. 2012, <http://en.wikipedia.org/wiki/Silane>
- ↑ http://web.princeton.edu/sites/ehs/labsafetymanual/cheminfo/silane.htm