Bowhunting is a tradition that many Americans take part in every year. There are many types of bows used for hunting such as the crossbow, long bow and the most popular, the compound bow. The compound bow is often preferred over long bows due to improved accuracy and arrow speed. Crossbows are often limited to those unable to use a traditional long or compound bows but still want to be able to hunt. A picture of a compound bow can be found in Figure 1.
A compound bow uses a system of cables and pulleys, known as cams, to operate the bow. When the string is pulled back, the cams rotate which puts stress on the limbs. As the limbs flex they store energy which makes the compound bow much more efficient than a traditional long bow. There are many important components on a bow including the arrow rest, sight, cables, and stabilizers. This new 3-D printing idea will concentrate on the arrow rest.
The arrow rest is designed to hold the arrow in a stationary and consistent position every single time the bow is pulled back. There are many different arrow rests currently on the market that function to hold the arrow before the shot. A very common rest, the drop-away rest, is depicted in Figure 2. This rest uses the drawing of the bow to raise a stabilizing arm that holds the arrow in a stationary position in the middle of the rest. The issues with these rests is that when the user is nocking and drawing back the bow, the arrow drags across the arrow rest creating extra noise. When firing the arrow, the idea is to minimize the contact of the arrow on the rest to reduce the chance of reducing the accuracy of your shot.

Touch-Less Arrow Rest[edit | edit source]
The touch-less arrow rest design we are proposing uses magnetic manganese doped zinc oxide (ZnMnO) which is 3-D printed on all sides of the rest. This creates a repulsive magnetic field that suspends the arrow head in the middle of the rest with no part of the arrow actually touching any part of the rest. This allows for a more accurate and consistent shot every time. The basis of the touch-less arrow rest is to create a magnetic repulsion around a magnetic insert in an arrow (the insert is what the tip of the arrow is screwed into). Once the bow is drawn, this will levitate the arrow to the center of the rest and the arrow will be free-floating in mid-air. An arrow rest is on the market today that levitates the arrow but does not utilize the use of semiconductors. It instead uses magnets that surround the arrow while using a magnetic tip to center the arrow. By using the ZnMnO, the heavy magnets used in current models of the device can be replaced by a smaller film of the semiconductor. The archery industry is always looking for ways to reduce the weight and increase the accuracy and mobility of bows to optimize the use for hunters. A few grams can make a big difference when combining several components. Also, ZnMnO will need a current running through it for the material to display magnetic properties. This will be done by connecting a battery with an on/off switch so when the bow is being stored, the magnetic semiconductor can be turned off.
Material Properties[edit | edit source]
Zinc Oxide is not naturally magnetic so the structure must be altered to obtain this property. One way to do this while maintaining it's semiconductor properties is through doping using Manganese to create a ferromagnetic transition metal.[1] In the field of materials science, ZnO is known to be a wide-bandgap semiconductor. This means that the material has electronic band gap that is much larger than a single electron volt (eV).
Synthesis[edit | edit source]
Option 1: Synthesis of Aqueous Solution[edit | edit source]
The first synthesis process of Mn doped ZnO powder starts with combining; zinc acetate (Zn(CH3COO)2*2H2O), manganese acetate (Mn(CH3COO)2*2H2O), and potassium hydroxide (KOH). Amounts of 1.975g of zinc acetate and 0.079g of manganese acetate are dissolved in 100mL of ultra pure water. Simultaneously, 0.28g of potassium hydroxide was dissolved in 10mL of ultra pure water. After 30 minutes of stirring the manganese acetate and zinc acetate mixture at room temperature, the potassium hydroxide solution is added drop-wise at 80-85oC with constant stirring. Precipitate will form after a 3 hour period and is collected and washed with ethanol and deionized water. The precipitate is then centrifuged at 3000rpm and dried for 6 hours at 50oC.[2]
Option 2: Microwave Irradiation[edit | edit source]
The second method in which to synethize zinc oxide (ZnO) nanopowders is by using a microwave irradiation system. The ZnO powders are to be prepared using the following equations:
Zn(NO3)2 + NaOH -- Zn(NO3)(OH) + NaNO3, (1)
Zn(NO3)OH +NaOH -- ZnO + NaNO3 + H2O, (2)
where the NaNO3 was washed away with excess water. The synthesis of ZnO is carried out by dissolving 1.89g of 99% pure zinc nitrate to make a 50 ml solution of 0.2M Zn(NO3). A manganese nitrate (Mn(NO3)2) dopant is added to the Zn(NO3)2) solution at a 5% concentration. During the stirring process, 50ml of 0.4 M NaOH is added dropwise to the mixture. This solution is then placed into a microwave where it is heated at 150W for 5 minutes. Once complete, the white precipitate that forms from the reaction is washed with several times with absolute ethanol and distilled water until the pH of the solution is 7. The final product is then dried at 70oC for four hours. Once complete the powder is ready for 3-D printing..[3]
Option 3: Solid State Reaction[edit | edit source]
The third process that can be used to synthesize a Mn doped ZnO is by the use of a solid-state reaction. This route uses polycrystaline solids of both MnO and ZnO and heats them to a high temperature around 1000-1500oC in order to cause a reaction. Using fine grained materials and extensively drying the powders before synthesis is key to a successful reaction. The ZnO:MnO weight ratio should be around 95% ZnO to 5% MnO to achieve an appropriately doped compound. The powders are to be ground using a mortar and pestle and acetone can be used to aid in the homogenization of the two crystals. This will form a paste and the acetone should be completely evaporated after 15-20 minutes. Since we are using larger quantities of powder, over 20g, a mechanical mixing process should be used and may take up to several hours to complete the evaporation process. Once the acetone has completely evaporated, the powder is ready for sintering..[4]
Testing procedures[edit | edit source]
XRD[edit | edit source]
To determine if you have Mn doped ZnO you can perform a x-ray powder diffraction (XRD) test that will show the intensity peaks of the compound.[5] From this the d-spacing of the element can be determined which will allow for the calculation of the lattice parameter and the crystal structure. Once these are determined, which is often given by computer analysis, the compound can be determined. To determine if the ZnO is doped with Mn several indicators can be determined by the XRD scan. First, the c lattice parameter decreases by several fractions of an angstrom. Also, the diffraction peaks are shifted 0.17o higher than a non-doped sample. This is caused by the smaller atomic radii Mn atom replacing Zn in the lattice.[6]
Purification[edit | edit source]
The purification process for the semiconductor is going to be completed before any actual synthesis takes place. The reagents are going to be purchased at purity above 99% which should help eliminate any concerns with impurities in the final product. An XRD scan will also be done on the powder before synthesis to verify that the desired elements are present and that there are minimal impurities in the sample. An acetone wash of the powder can also be completed to help remove unwanted impurities from the sample.
Manufacturers/Supplier[edit | edit source]
- Metallurgical Plant ChZZ Oskid Ltd
- Forbes Pharmaceuticals
- Triveni Aromatics And Perfumery Private Limited
- SIGMA-ALDRICH
Cost of Raw Materials[edit | edit source]
| Material (MSDS) | Cost (US$/g) |
|---|---|
| Manganese acetate MSDS | $3.975[7] |
| Zinc Acetate MSDS | $0.155[8] |
| Manganese (2)nitrate tetrahydrate MSDS | $0.149[9] |
| Zinc nitrate hexahydrate MSDS | $0.145[10] |
| Sodium Hydroxide MSDS | $21.7/L[11] |
| Zinc Oxide MSDS | $0.006[12] |
| Manganese(2) Oxide MSDS | $0.09[13] |
Total Cost of Arrow-Rest[edit | edit source]
One of the factors that affected what synthesis method we are planning to pursue came down to the cost of the raw materials needed to produce the rest. After some calculations it was determined that the total volume of our part was 44.06 cm3. Also, a percent yield for the reaction was not found, for cost estimation purposes a 90% yield was used. The amounts of reagents needed are: ZnO = 258.4g MnO = 13.6g
The total price of materials for each touch-less arrow rest comes out to be $2.78. This price is a little lower than we were expecting. If the product did not meet the desired requirements we would go back and try to find a more pure form of the reagents used. This would increase the cost of the part but would also reduce the number of impurities which would positively affect the performance. This does not take into account the power used in processing or other production costs. The most inexpensive arrow rest at Bass Pro Shop was priced at $34.99[14] and a standard drop down rest was priced at $109.99.[15] This leaves quiet a bit of overhead for energy costs per unit.
3-D Printing[edit | edit source]
There are multiple 3-D printing methods that could be used with our materials. They include Electron Beam Freeform Fabrication (EBF3), Direct Metal Laser Sintering (DMLS), and Selective Laser Sintering (SLS). All three methods are capable of printing ZnO, but the type of 3-D printing that appears most effective for this semiconductor is Selective laser sintering. This method is most useful because ZnO often comes in or can be synthesized in a powder form, which can be 3-D printed using this process.[16]
Selective Laser Sintering (SLS)
The concept of Selective Laser Sintering (SLS) as a 3D printing method has been around since the mid 1980's. The method uses a high powered laser to generate a product. The laser is able to fuse small particles together and does this continually, layer by layer until the entire part is complete. A thin layer of powder layed down and heated to reduce the temperature change the laser has to produce to fuse the powder. The computer sends in the dimensions of the CAD drawing and the laser follows this drawing and only melts the powder in the appropriate locations. Once the layer is fused, a chamber to the left of the powder bed raises and a roller applies another uniform layer of powder on top of the powder bed and the process repeats until the part is complete. A depiction of this process can be found in Figure 3 and was taken from the Wikipedia page describing Selective laser Sintering.[17]

Selective Laser Sintering can either work with single-component or two-component powders with the latter being the most common. This method is able to produce parts from a variety of powdered materials which makes gives it an edge over similar 3D printing methods. The process is also adaptive in that it can perform a variety of functions such as full or even partial particle melting on many common powders. Zinc Oxide has similar properties to materials that are commonly used in SLS and it is anticipated that there will be minimal disturbances to the process using this compound.[18]
The table bellow shows the comparison of ZnO versus other commonly used elements for selective laser sintering like steel and titanium. This shows that ZnO has similar properties and in the appropriate range for useable elements.
Best in Class Materials[edit | edit source]
| Material | Melting Temp (C) | Density (g/cm^3) | Young's Modulus (GPa) | Band Gap (eV) | Crystal Structure |
|---|---|---|---|---|---|
| ZnO | 1975[19] | 5.606[20] | 31 (3 point bend test)[21] | 3.3[22] | hexagonal wurtzite[23]/cubic zincblende[24] |
| Steel | 1370[25] | 7.75-8.05[26] | 200 (structural steel)[27] | NA[28] | BCC/FCC[29] |
| Titanium | 1668[30] | 4.506[31] | 116[32] | NA[33] | HCP[34] |
Benefits of 3-D Printing[edit | edit source]
The touch-less arrow rest can be achieved using regular magnets,[35] but a 3-D printable semiconductor with magnetic properties willreduce the overall weight to the bow, allowing the archer to be more accurate with their shot. This will also allow the shooter to maintain a more steady hand while taking the shot. 3-D printing is also very desirable for the arrow rest because ZnMnO, along with the 3-D printing process is fairly inexpensive allowing for the rapid mass production of this product. Depending on the synthesis process used, the total cost of the part can be significantly less than other arrow-rests currently on the market.
In-Situ Analysis[edit | edit source]
The main property of our semiconductor that is going be measured is the magnetic force that it produces. To test this in-situ may not be plausible without the possibility of disrupting the production process. The best way to test this material as early as possible is to test the magnetization of the Mn doped ZnO soultion before it is 3-D printed. This can be easily tested by one of two ways. You can run the solution through a vibrating sample magnetometer (VSM) which will induce a current through the solution and show the magnetization characteristics of the sample. You can also check through a less accurate method and simply hold a magnet up to the solution and see if it has a visible reaction. The justification for in-situ analysis is clear when the part being made is of high cost and importance. Since we are making such cost effective parts with our design, roughly less than $10, it is not necessary to invest a large amount of capital to perform in-situ analysis. We would plan to rely on testing the powder before sintering and testing the completed part after manufacturing for performance verification. Though using an in-situ method would not be in our plans, it is plausible that conducting a VSM test would give us the data we desired in-situ.
Workflow Diagram[edit | edit source]
Fig 4: Workflow Diagram from Dia
The solid state reaction would be the optimal synthesis path due to ease of preparation and cost effectiveness of this process. The microwave irradiation would be the next best synthesis to try if the solid state reaction did not result in the proper powder with the necessary magnetic properties. The chemical reaction would be the last method due to the difficulty to reproduce and the cost of many excess materials involved in synthesis.
Prototype Design[edit | edit source]
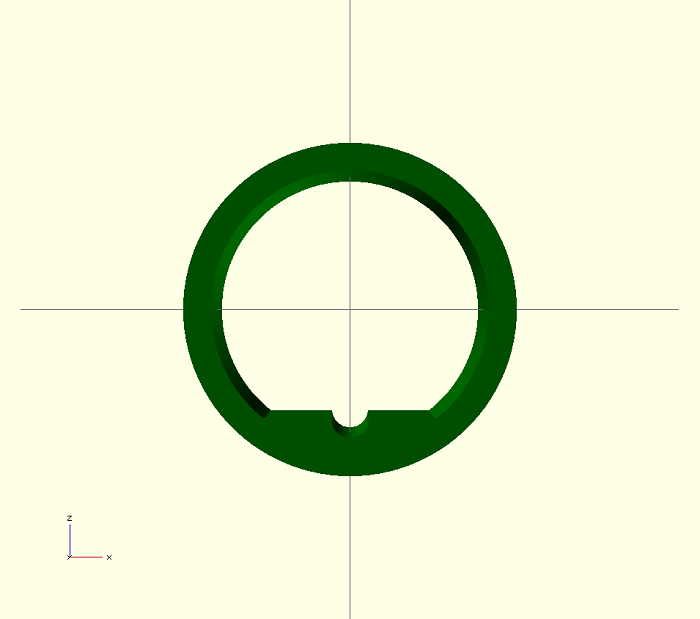
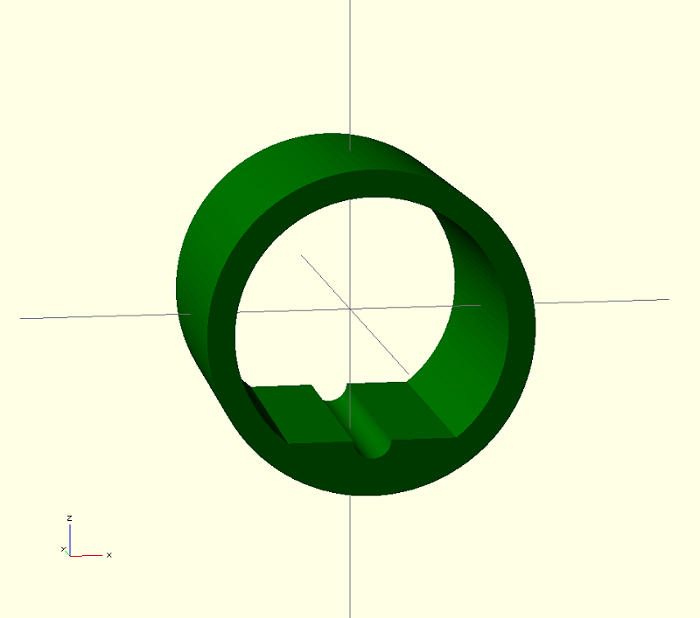
Figures 5 and 6 show the prototype model of the Mn-ZnO touch-less arrow rest. This design allows standard arrow vanes to move through the rest without being hindered and throwing the arrow off course. A cylindrical groove is designed into the bottom of the rest which allows the arrow to sit and slide in the groove prior to being drawn. Arrows will be fitted with a magnetic insert were the arrow head meets the shaft. Because of the circular design, once the bow is drawn and the magnetic insert is within the rest it will appear to float. This will increase the accuracy of the bow by eliminating the contact of the arrow on the rest during the shot
Fig 5: OpenSCAD model of the semiconductor part of the touch-less arrow rest. This model is in a mm scale.
Fig 6: The outside diameter of the rest is 30mm and the inside diameter is 25mm.
SCAD Code
// substract1.stl // Units: mm
difference() {
cylinder(h=58, r=100, center = true, $fn=100);
cylinder(h=60, r=30, center = true, $fn=100);}
// ZnORing.stl //Units: mm
color([0,.4,0]) difference() {
cylinder(h=50.8, r=30, center = true, $fn=100);
cylinder(h=52.8, r=25, center = true, $fn=100);}
//Final Assembly of Touchless Arrow Holder // Created by: Peter Tropper //The units for this project are mm.
color([0,.4,0]) rotate ([90,90,0]) union(){ import("ZnORing.stl"); difference () { translate ([26,0,0]) {cube (size = [12.7,100,50.8], center=true);} import("subtract1.stl"); translate ([19.5,0,0]){cylinder (h=55, r=3.5, center = true, $fn=100);}}}
Circuit Design
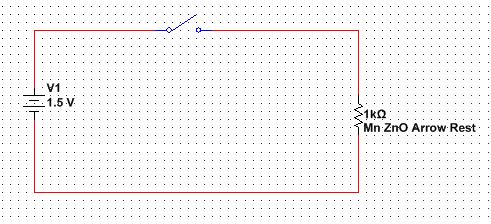
Figure 7 shows a circuit design for the touch-less arrow rest. This circuit is simple, which for this application is good. The saturation point for Mn-ZnO needs to be reached for permanent magnetism to be reached. Further testing would be required to determine the type of battery needed for this application. The current design employs a AAA battery.
Fig 7: Simple circuit design for the touch-less arrow rest.
References[edit | edit source]
- ↑ http://search.proquest.com/docview/852004435?accountid=28041 Dilute Magnetic Semiconductors and Oxides.
- ↑ http://iopscience.iop.org/2043-6262/3/3/035005/pdf/2043-6262_3_3_035005.pdf Synthesis, characterization and properties of Mn-doped ZnO nanocrystals
- ↑ http://iopscience.iop.org/1742-6596/187/1/012020/pdf/1742-6596_187_1_012020.pdf
- ↑ http://en.wikipedia.org/wiki/Solid-state_reaction_route#cite_ref-2
- ↑ http://web.archive.org/web/20200201183748/http://www.ccp14.ac.uk:80/poster-talks/phase-id-1999/html/phaseid.htm Hints on Phase Identification Using Powder X-ray Diffraction
- ↑ http://iopscience.iop.org/2043-6262/3/3/035005/pdf/2043-6262_3_3_035005.pdf Synthesis, characterization and properties of Mn-doped ZnO nanocrystals
- ↑ http://web.archive.org/web/20171105120805/http://www.sigmaaldrich.com:80/catalog/product/aldrich/215880?lang=en®ion=US Cost of Manganese acetate
- ↑ http://web.archive.org/web/20160614003059/http://www.sigmaaldrich.com:80/catalog/product/sial/96459?lang=en®ion=US Zinc Acetate
- ↑ http://web.archive.org/web/20160503163803/http://www.sigmaaldrich.com:80/catalog/product/sial/63547?lang=en®ion=US Manganese(II) nitrate tetrahydrate
- ↑ http://web.archive.org/web/20170919075259/http://www.sigmaaldrich.com:80/catalog/product/aldrich/230006?lang=en®ion=US Zinc nitrate hydrate
- ↑ http://web.archive.org/web/20160916074855/http://www.sigmaaldrich.com:80/catalog/product/fluka/38215?lang=en®ion=US Sodium hydroxide concentrate
- ↑ http://eng.lorini.biz/zinc/ossidozinco.htm
- ↑ http://web.archive.org/web/20170629024936/http://www.sigmaaldrich.com:80/catalog/product/aldrich/377201?lang=en®ion=US
- ↑ http://www.basspro.com/New-Archery-Products-QuikTune-800-Arrow-Rest/product/33714/?cmCat=CROSSSELL_PRODUCT
- ↑ http://www.basspro.com/Ripcord-Arrow-Rests/product/79501/?hvarAID=shopping_googleproductextensions&om_mmc=shopping_googleproductextensions&kpid=79501
- ↑ http://en.wikipedia.org/wiki/3D_printing
- ↑ http://en.wikipedia.org/wiki/Selective_laser_sintering Selective laser sintering
- ↑ http://en.wikipedia.org/wiki/Selective_laser_sintering Selective laser sintering
- ↑ http://en.wikipedia.org/wiki/Zinc_Oxide Zinc Oxide
- ↑ http://en.wikipedia.org/wiki/Zinc_Oxide Zinc Oxide
- ↑ http://iopscience.iop.org/0957-4484/18/20/205503/pdf/0957-4484_18_20_205503.pdf
- ↑ http://en.wikipedia.org/wiki/Zinc_Oxide Zinc Oxide
- ↑ http://en.wikipedia.org/wiki/Zinc_Oxide Zinc Oxide
- ↑ http://en.wikipedia.org/wiki/Zinc_Oxide Zinc Oxide
- ↑ http://education.jlab.org/qa/meltingpoint_01.html Steel Properties
- ↑ http://en.wikipedia.org/wiki/Steel Steel
- ↑ http://en.wikipedia.org/wiki/Steel Steel
- ↑ http://en.wikipedia.org/wiki/Steel Steel
- ↑ http://en.wikipedia.org/wiki/Steel Steel
- ↑ http://en.wikipedia.org/wiki/Titanium Titanium
- ↑ http://en.wikipedia.org/wiki/Titanium Titanium
- ↑ http://en.wikipedia.org/wiki/Titanium Titanium
- ↑ http://en.wikipedia.org/wiki/Titanium Titanium
- ↑ http://en.wikipedia.org/wiki/Titanium Titanium
- ↑ http://web.archive.org/web/20141220055930/http://www.doubletakearchery.com:80/air-rest/faqs.htm Touchless Arrow Rest
Contact Information[edit | edit source]
Email:kmikeda@mtu.edu
Email:clheitin@mtu.edu
Email:pdtroppe@mtu.edu