Currently the market for solar cells depends on the manufacturing cost, module efficiency cost, and the cost of electricity being the biggest factor for solar cells. The second generation thin film solar cell, such as CdTe's manufacturing cost is $0.98/Wp and a 10% module efficiency.[1] The third generation solar cells (CdSe Quantum Dots) are still in the lab phase, but are suppose to cost less to manufacture, with a higher module efficiency, and sell at a lower price. The first and second generation solar cells have a maximum theoretical conversion efficiency of 31%, whereas the thurd generation's maximum theoretical conversion efficiency is 66%, which is more than double the effciency.[2] When the third generation solar cells like CdSe Quantum Dots Heterojunction solar cells start to be manufactured, they will surpass their predecessors (1st and 2nd generation solar cells) and be the desired solar cells on the market. The solar powered purification tank can be in the market for purifying contaminated drinking water and remove salt from salt water. There is a big demand for clean water in the world. Approximately 740 million people don't have a clean water source available[3] and approximately 3.4 million people die each year from contaminated water, sanitation, and hygiene problems.[4] Being able to have cheap available clean water can help reduce the amount of deaths and raise the standard of living for people who don't have access to clean water. If the solar powered purification tank can be made to be affordable for people in the regions of the world that don't have clean water, then human standard of living on Earth can be raised.
Advantages of Quantum Dots Over Current Solar cells[edit | edit source]
- Don't rely on a single p-n junction configuration
- Tandem cells-stacks of p-n junctions structures
- Theoretical energy conversion efficiency of up to 66%[5]
- Optical and electronic properties can be tweaked by changing the size of Quantum Dots[6]
- Able "to inject electrons to a wider band gap material, such as TiO2"[7]
- Higher light to energy efficiency
- Easy to manufacture
- Low cost
- Capable of layer-by-layer deposition[8]
- Theoretical Energy Conversion Efficiency of 66%[9]
- Quantum dots can be produced in stable colloidal suspensions, which help to break barriers in production and implementation that were in place because of traditional crystalline growth procedures
- Multiple Excitation Generation (meaning a single photon results in multiple electron-hole pairs), allows for QD solar cells to theoretically surpass the Shockley–Queisser limit
- The Quantum Confinement effect results in the variation of the band gap of nanocrystals as a function of size. This will allow for thin film layers to be tailored to absorb certain wavelengths of the solar spectrum
How CdSe Quantum Dots Work[edit | edit source]
Semi-conductive nano-particles also known as Quantum Dots (QDs) absorbs light in solar cells. The characteristics of QDs that are needed for transferring the absorbed light's energy is its conduction and valence bands of the QDs permit electron injection and hole transportation through to the metal oxide and metal layers, which the QDs is between.[10] The amount of light absorbed by the Quantum dots depends on its thickness if it is too thick, the collection of photogenerated charge carriers is incomplete, while too-thin QD layers show poor light harvesting.[11] The QDs size also plays a factor in its performance when open circuit voltage, fill factor and photocurrent decrease with increasing the QD size; however, inner-particle electron transfer is facilitated in films made of the larger QDs.[12] Electrons and holes move faster "by one or two orders of magnitude with an increase in QD diameter.[13]
These solar cells don't rely on single p-n junction design, but uses tandem cells or multi-junction solar cells with a stack of p-n junctions of low-dimensional semiconductor structures.[14] The p-n junction stacks can have different Eg, thus covering a very wide range of the solar spectrum, thus increasing the theoretical energy conversion efficiency from 31% to 66%.
Project goals[edit | edit source]
Our goals for this project are to learn about two current technologies and their applications: semiconductors and 3D printing. While brainstorming for the project, we had various ideas, including piezoelectric pressure sensors for car bumpers and football helmets, solar powered watches and cell phone cases, but settled on an idea that we thought could actually be helpful in peoples lives (once the technology catches up).
The idea is a self contained water purification tank for areas of the world that lack clean drinking water. The eventual goal of the product is an easily 3D printable standard design that can be slightly modified for different locations and populations. A 3D printer, capable of printing the tank, would be sent to geographical locations where fresh water is scarce and there is a need for multiple units. In turn, shipping costs would be minimized by the nearly on-site manufacturing of the tank. The active materials of the tank's thin film would need to be imported, but it may be possible to utilize thermoplastics found on site such as milk jugs and plastic bags (or even clay) in the tank's structure.
As is evident from the color coded map of water availability, this product could be useful to the people of a variety of locations, ranging from the Western US, to Peru, to Central and Southern Africa, to areas of the far East. It would also be appropriate for survivalists, campers, and sailors (or anyone who spends extended periods of time on saltwater).
CdSe Nanocrystal "Ink" Synthesis[edit | edit source]
There are a variety of techniques to synthesize colloidal solutions of Cadmium Selenide. However, long, bulky ligands are also formed (and attached to the nanocrystals) during synthesization. In the reaction these Native Ligands are used advantageously, to control crystal growth, nucleation, and to prevent nanocrystals from agglomerating in solution. Once it is time for the CdSe nanoparticles to perform, for instance in a semiconducting thin film, the ligands act as insulation to the nanocrystals and destroy the carrier mobility of the semiconductor. For this reason, in conjunction with the possibilities for new nanoparticle-fueled semiconductors, much research is currently underway with the focus of Native Ligand Exchange. Click Here for more info on techniques for Native Ligand Exchange.[15]
The Ligand Exchange technique that will be highlighted here, involves a colloidal exchange of Native Ligands for the Thiocyanate precursor, the 1,2,3,4-thiatriazole-5-thiolate anion (TTT-). CdSe nanocrystals with TTT- ligands (called CdSe(TTT)) have long term stability in solution, which would allow for synthesis of large volumes of nanoparticles without the need for immediate printing onto the water purification system. Upon mild heating of >100C, TTT- readily thermolyzes into to the small, minimally insulating ligand Thiocynate, which is commonly used in the formation of high quality nanocrystal semiconductor films.[16]
Synthesis of Native Ligand CdSe nanocrystals, in solution:
- Mix 3.5g CdCO3, 30g Stearic Acid, and 30g TOPO, stirring at 100C for 90 minutes under flowing N2
- Stop flow of N2, and hold at 360C for 60 minutes
- In a different container, dissolve 2.3g Selenium powder in 30ml TOP under flowing N2
- Quickly add the Selenium/TOP solution to the initial solution (containing the dissolved CdCO3), while stirring rapidly
- Immediately after, quickly mix in 30mL Octadecene
- Hold this solution at constant temperature for 2 minutes, then quench the system using the air flow from a fume hood
- When the solution has cooled to nearly room temperature, add 30mL of toluene (previously nitrogen sparged)
- Transfer equal volumes of the solution to centrifuge tubes for centrifugation
- Add equal volumes of MeOH and EtOH to the centrifuge tubes, for total volumes of 60mL and 66mL MeOH and EtOH, respectively
- Centrifuge down the nanocrystals in solution at 6000 rpm for 6 minutes
- Remove and discard residual supernatant
- Wash the nanocrystals by re-dispersing them in 120mL Toluene, followed by 150mL EtOH to assist in flocculation
- Centrifuge the solution at 6000 rpm for 1 minute, then discard supernatant
- Repeat this washing procedure up to 6 additional times, while decreasing the volume of dispersant and flocculant to a minimum of 60mL each
- Perform the final redistribution in 60ml Toluene
- Filter the final dispersion through a 450nm syringe filter to separate any agglomerates that may have formed
- Store the product in the dark at 10C
Note: This process typically yields around 3.3g of CdSe nanocrystals. "Scaling-up" in production is possible given the nature of the procedure.[17]
CdSe nanocrystal Native Ligand Exchange using the TTT- anion:
Synthesis of ligand-donor molecule through the cation exchange of (NH4)2SiF6 and NaTTT
- Preparation of NaTTT solution
- Dissolve 500mg NaN3 in 2ml distilled water and 2ml nPrOH (nPrOH added to aid in homogeneity)
- While limiting light exposure, add 0.50ml CS2, and agitate vigorously for 40 minutes to ensure a single phase
- Preparation of NH4TTT solution
- Dissolve 695mg (NH4)2SiF6 in 5ml distilled water
- Over 8 minutes and with vigorous agitation, slowly and continuously add the (NH4)2SiF6 solution to the NaTT solution
- Add 20ml nPrOH
- Centrifuge the resulting NH4TTT solution with 6000rpm for 1 minute, and filter though a 450nm syringe filter
- Store the solution in a cold (7-8C), dark place to secure colloidal stability for >2 weeks
Note: In the step above, it is important to limit exposure of heat and photons, to ensure that TTT- does not prematurely decompose into SCN-
TTT- - NL Ligand Exchange
- Dilute 2.5ml of the NH4TTT solution with 10ml nPrOH
- Using around a 0.0388 wt/vol% of CdSe nanocrystals dispersed in Toluene, inject 2.5ml into the diluted NH4TTT solution
- Mix solution, centrifuge at 6000rpm for 0.5 minutes, and remove supernatant without drying crystals
- Redisperse the CdSe(TTT) nanocrystals in 5ml Propylene Carbonate, and exchange remaining Native Ligands by adding another 2.5ml NH4TTT solution
- Allow solution to stand in the dark for 30 minutes
- Add 30ml Dimethoxymethane, and repeat the centrifugation and supernatant removal process
- Again redisperse the solid in 5ml Proplyene Carbonate, and filter through a 450nm syringe to remove any precipitates
- Again wash the CdSe nanocrystals in 30ml Dimethoxymethane, and repeat the centrifugation and supernatant removal process
- Prepare the nanacrystals for a final distribution by dispersing in 5ml Propylene Carbonate, and adding 80ml Dimethoxymethane and 20ml Pentane to facilitate flocculation
- Centrifuge solution at 6000rpm for 5 minutes, and remove supernatant
- Perform the final redistribution in a chosen dispersant (or mixture of dispersants) that is/are appropriate for the printing application. Chief considerations should be liquid viscosity, dispersibility, evaporation rate, and safety hazards. The dispersant selection process should be analogous to the selection process for spin coating of electronic wafers
- Solvents that are know to have high dispersibility with CdSe(TTT) nanoparticles include Propylene Carbonate, Sulfolane, N,N-Dimethylformamide, Dimethyl Sulfoxide, N-methylformamide, and Tetramethylurea
- For this illustration, the final dispersant is 1ml DMF
- Prepare colloidal suspension with bubbling N2 to evaporate residual Dimethoxymethane, and centrifuging (6000rpm, 0.5 minutes) and decanting to remove any small agglomerates
- Store the CdSe(TTT) colloid in a cool (7-8C) dark place
Note: This process typically yields a CdSe colloidal suspension with a concentration of around 81 mg/ml. "Scaling-up" in production is possible given the nature of the procedure.[18]
Required Chemicals (click for MSDS)
- Cadmium Carbonate (CaCO3)

- ~4.06$/gram
- Stearic Acid

- ~3.30&/gram
- Tri-n-octylphosphine oxide (TOPO)
- ~0.50$/gram
- Selenium Powder
- ~4.56$/gram
- Tri-n-octylphosphine (TOP)

- ~1.01$/mL
- Octadecene

- ~32.40$/L
- Toluene
- ~26.20$/L
- Methanol
- ~30.75$/L
- Ethanol
- ~107.17$/L
- Sodium Azide (NaN3)

- ~186.25$/kg
- Distilled water
- n-propanol
- ~58.75/L
- Carbon disulfide

- ~231.5$/L
- Ammonium Hexafluorosilicate
- ~2.70$/gram
- Propylene Carbonate

- ~67.75$/L
- Dimethoxymethane
- ~21.78$/L
- Pentane
- ~34.50$/L
- N,N-Dimethylformamide (DMF) or chosen dispersant
- ~40.11/L
Note: All pricing quotes assume bulk orders from Sigma Aldrich
Characterization of CdSe[edit | edit source]
While there are many techniques to perform a quantitative analysis, here we will focus on two of the more common tools for material analysis: X-Ray Diffraction (XRD) and Ultraviolet-visible spectroscopy (UV-Vis) analyses.
X-Ray Diffraction Analysis-XRD is a common tool used to analyze and characterize different materials and their properties. The procedure sees a small powder or thin film sample loaded into an X-Ray diffractometer and then exposed to X-Rays. The angle at which the incident rays meet the sample and the angle at which the diffracted rays are caught by the detector are changed periodically during the test. This is because different materials will have different positions where the energy diffracted toward the detector is at its highest, these will form the peaks we see on diffractograms. These peaks, called characteristic peaks, are primarily used in the identification of a material and its crystal structure.
The figure to the left is a diffractogram of a sample of CdSe Quantum Dots given by an XRD test along with an overlay of the diffraction pattern of CdSe provided by the International Center for Diffraction Data (ICDD). The ICDD collects and maintains Powder Diffraction Files (PDFs) on most known elements and compounds, as the location and magnitude characteristic peaks found in XRD are unique to a particular material, identifying unknown materials and compounds is easy with an accurate enough diffractogram. The CdSe diffractogram to the left lines up pretty well with the CdSe PDF with respect to potential impurities or human/machine error. Particle size, interplaner spacing, and a number of other properties of a sample can be taken from XRD, using equations like the the Bragg Equation and the Scherrer Equation and other techniques as well. This approach would be most effective if a sample of ink was printed into a thin film to be used in a diffractometer. It would confirm whether or not the tested batch could or should be used well before it was used during manufacture.
Ultraviolet-visible spectroscopy tests examine the absorption or reflective spectroscopy of a material over the ultraviolet-visible spectral region. The test itself bombards a sample of a material with light of varying wavelengths in a spectrophotometer, as it's exposed to different light the spectrophotometer monitors the intensity at which the sample absorbs (or reflects) the light at these different wavelengths. And with the help of logging software it can produce spectral readouts like the one below:
UV-Vis spectroscopy tests could be performed after the synthesis of the CdSe Ink described in the sections above. And the desired result would be like the spectral readout above, where most light absorbed is either in or close to the UV spectrum and the intensity of light absorbed dropped significantly outside that range. This test needs to be performed on the ink to be used before printing to ensure that is capable of catching and using sunlight. Using the information from this readout, it is also possible to calculate the material's Band Gap Energy. Using Plank's Relation, the energy required to excite electrons through the material's band gap can be calculated if the maximum wavelength of light capable of being absorbed is also known.
Where E is the Band Gap energy, h is Planck Constant, c is the Speed of Light, and λ is Wavelength. As already stated, the wavelength must be known. Looking at the spectral readout, you can see that while at 400 nm the material is absorbing ambient light at a high intensity but drops shortly afterward. Just looking at the readout, one could estimate that between 500 and 550 nm is where the bottom of the bell curve starts, and that would be the wavelength to be used in the calculation for energy.
Design[edit | edit source]
The Quantum Dot Heterojunction Junction Solar Cell
There are several different cell architectures that are currently being used in Colloidal Quantum Dot (QCD) solar cell research; they include quantum dot sensitized cells, Schottky Junction cells, and a variety of heterojunction based cell designs. Current research efficiencies for QCD solar cells are around 3-5%, and show signs of steady increases to come.[19]
For this application, the solar cell will use a semiconductor-semiconductor heterojunction as it's effective p-n junction. Titanium Dioxide is a metal oxide that is commonly used as a transparent film with n-type semiconductor properties due to oxygen vacancies in its lattice and the presence of negatively charged charge carriers. This film material is intentionally chosen with a high band gap (~3.5eV, depending on nanoparticle size and preparation technique),[20] and subsequently allows the majority of photons to pass through into the layer of colloidal CdSe nanocrystals. TiO2 nanoparticles are available for purchase from chemical companies (for example Sigma Aldrich, ~1.13$/gram, 21nm), and can be redispersed in a chosen of dispersant. Once the majority of incoming photons pass through the metal oxide layer, they are absorbed by the thin film of CdSe nanocrystals, with a lower (and much easier to excite) band gap. The thickness of these layers is typically 100-300nm. See corresponding plot of the CdSe nanocrystal band gap as a function of size.[21][22]
Movement of electrons generated from the CdSe nanocrystal layer (p-type) to the TiO2 layer (n-type) is made possible by the induced electric field, created by the depletion layer that results from contact between the two semiconductors (and charge carrier transfer from n-layer to p-layer). Free electrons are then transferred out of the metal oxide and into a transparent electrode (usually Indium Tin Oxide), while holes move in the opposite direction toward a metallic electrode (usually Ag) backing. See the corresponding plot of cell structure and energy levels for a visual representation of process.
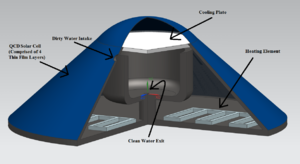
Water Tank Design The unit is essentially a water tank, encapsulated with a QCD solar cell, that produces energy used for purifying drinking water. The electricity created from the solar cell surface of the tank is sent through a simple resistor heating element found at the bottom, which boils the dirty water found inside. A simple battery system, or a series of capacitors, could also be used to store created charge that isn't immediately needed. The water evaporates, leaving the outer ring of the reservoir, and condensates at on the inverted cone top portion of the design, which is made of a polymer with very low thermal conductivity. The condensated water then rolls to the tip of the cone, and falls into the clean water reservoir, then out of the tank.
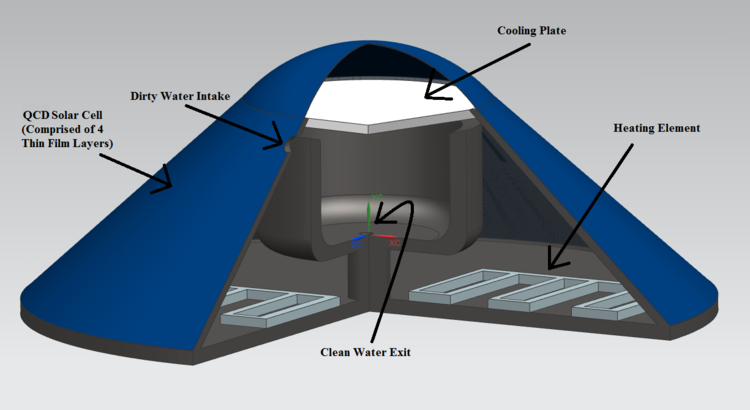
The size of the unit can be scaled up or down depending on the volume of clean water that is needed. The angle of inclination should also be varied, and set to the optimum angle given the latitude of its intended location. For example, a 55 gallon tank, with an intended location of Arizona and an optimum inclination of angle of around 30 degrees, would have a radius and height of around 70cm and 40cm, respectively.
3D Printing Advantages The manufacturing advantages to 3D printing the water purification system lie in the design and function of the unit, as well as the nature of the semiconductor nanocrystals. Via 3D printing, the conically shaped tank, made out of a polymer with a melting point higher than 100C (like nylon or reinforced HDPE) can be easily manufactured with common extrusion printing techniques. The curved QD solar cell can then be printed onto the conical surface of the tank, using dispersant evaporation techniques analogous to those used in spin coating. This would allow for uniform layer by layer deposition and the ability to segment cells as needed. The fact that deposition of the CQD thin films doesn't rely on extremely hot melting temperatures allows the tank to be made out of relatively inexpensive polymers, compared to the glass substrates that are usually associated with solar cells. Possibly the most convincing advantage to 3D printing is the fact that it allows for manufacturing of the unit in the geographical location where it is needed. The manufacturing costs are equal to the cost of the 3D printer, and the manufacturing "plant" is located where needed.
Costs[edit | edit source]
There are various aspects of the design that still need to be explored before an accurate estimation of production costs can be determined. Prices for bulk orders of the chemicals needed for synthesis of the of the active nanocrystals can be found in the corresponding "Synthesis" and "Design" sections. Data needs to be collected regarding the concentration of nanoparticles in the colloidal "Ink", as well a the dispersant (or mixture of dispersants) that is chosen. Work also needs to be done on reducing the cost of the tank structure, which is optimally composed of a relatively high bond strength polymer, like Taulman 618 Nylon, which has an extrusion temperature of ~250C, and a price of $19.95/lb through Amazon.com. The price can potentially be decreased by including recycled thermoplastics, such as milk jugs and plastic bags, that could be found at the location of printing. An outline of additional work that needs to be done regarding material alternatives can be found in the following section of this page.
Discussion[edit | edit source]
Next steps[edit | edit source]
There are still a few obstacles that need to be investigated before a 3D printable, self-contained, solar powered, water purification system can be materialized without the need for high melting temperature extrusion printing processes. Printing of the two electrodes needed to export the charge created from the CQD thin film (composed of Indium Tin Oxide and Silver) would require the ink and the extrusion nozzle to be of temperatures in excess of the melting temperature of the tank "shell". The same problem occurs when attempting to print the resistive heating elements needed to boil the dirty water located in the outer reservoir of the tank, which are typically made of a metal alloys. Work needs to be done to find new materials, possibly conductive polymers, that lend themselves to 3D printing without the need for high temperatures. The new materials also need to satisfy the internal properties required for the components to do their respective jobs (for example resistivity of the the heating coils, work function and conductivity of the electrodes).
The efficacy of this product will also increase with advancements of Quantum Dot photovoltaics. Chiefly, less expensive nanocrystal suspensions are currently being investigated. Colloidal suspensions of Zinc Phosphide nanocrystals have already been produced, which can be made from naturally abundant elements.[23] More advanced solar cell architectures will also help to improve CQD solar cell efficiencies, like multilayer cells that can absorb much more of the solar spectrum. This is accomplished through varying the size of nanocrystals in each consecutive layer. Due to the quantum confinement effect in nanoparticles, this varying of size is accompanied by a predictable change in the band gap of the thin film.
Conclusions[edit | edit source]
Once 3-D printing CdSe Quantum Dot Heterojunction solar cell becomes reality, the solar powered water purification system can be made affordable for exporting into regions of the world that have a lack of clean drinkable water. Quantum Dot solar cells should be used, because theoretical conversion efficiency is more than double the theoretical conversion efficiency of single crystal solar cells. Since quantum dots will have a higher energy conversion efficiency than the other types of solar cells, thus it is the best solar cell for heating purposes. Theoretically, 3-D printing of quantum dots should be the best way to produce layered quantum dots on all shapes of surfaces and can make the water tank into a water purification unit with the help of a heating element.
References[edit | edit source]
- ↑ Hillhouse, Hugh., and Beard, Matthew. "Solar cells from colloidal nanocrystals: Fundamentals, materials, devices, and economics", Elsevier 14 (2009):245-249, 20013. Web.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ UNICEF. "Progress on Drinking Water and Sanitation: 2012 Update", UNICEF and World Health Organization. (2012)
- ↑ Pruss-Ustun, A., Bos, R., Gore, F., Bartrum J."Safer Water, Better Health: Costs benefits and sustainability of interventions to protect and promote health", World Health Organization, Geneva (2008)
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Webber, David H., and Richard L. Brutchey. "Nanocrystal Ligand Exchange with 1,2,3,4-thiatriazole-5-thiolate and Its Facile in Situ Conversion to Thiocyanate." Dalton Transaction 41.26 (2012): 7835-838. Print.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Webber, David H., and Richard L. Brutchey. "Ligand Exchange on Colloidal CdSe Nanocrystals Using Thermally Labile Tert-Butythiol for Improved Photocurrent in Nanocrystal Films." Journal of the American Chemical Society 134 (2011): 1085-092. Print.
- ↑ Webber, David H., and Richard L. Brutchey. "Nanocrystal Ligand Exchange with 1,2,3,4-thiatriazole-5-thiolate and Its Facile in Situ Conversion to Thiocyanate." Dalton Transaction 41.26 (2012): 7835-838. Print.
- ↑ Webber, David H., and Richard L. Brutchey. "Ligand Exchange on Colloidal CdSe Nanocrystals Using Thermally Labile Tert-Butythiol for Improved Photocurrent in Nanocrystal Films." Journal of the American Chemical Society 134 (2011): 1085-092. Print.
- ↑ Webber, David H., and Richard L. Brutchey. "Nanocrystal Ligand Exchange with 1,2,3,4-thiatriazole-5-thiolate and Its Facile in Situ Conversion to Thiocyanate: Supplementary Information." The Royal Society of Chemistry, Electronic Supplementary Material (ESI) for Dalton Transactions (2012): n. pag. Print.
- ↑ Nikolenko, L. M., and V. F. Razumov. Colloidal Quantum Dots in Solar Cells. Russian Chemical Reviews 82.5 (2013): 429-48. Print.
- ↑ Vijayalakshmi, R., and V. Rajendran. "Synthesis and Characterization of Nano-TiO2 via Different Methods." Archive of Applied Science Research 4.2 (2012): 1183-190. Web.
- ↑ Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- ↑ Nikolenko, L. M., and V. F. Razumov. Colloidal Quantum Dots in Solar Cells. Russian Chemical Reviews 82.5 (2013): 429-48. Print.
- ↑ Luber, Erik., Mobarok, M., and Buriak, J. "Solution-Processed Zinc Phosphide (α- Zn3P2) Colliodal Semiconducting Nanocrystals for Thin Film Photovoltaic Applications ", National Institute for Nanotechnology, p.A-K. www.ascnano.org. 2013.
- Webber, David H., and Richard L. Brutchey. "Ligand Exchange on Colloidal CdSe Nanocrystals Using Thermally Labile Tert-Butythiol for Improved Photocurrent in Nanocrystal Films." Journal of the American Chemical Society 134 (2011): 1085-092. Print.
- Webber, David H., and Richard L. Brutchey. "Nanocrystal Ligand Exchange with 1,2,3,4-thiatriazole-5-thiolate and Its Facile in Situ Conversion to Thiocyanate." Dalton Transaction 41.26 (2012): 7835-838. Print.
- Webber, David H., and Richard L. Brutchey. "Nanocrystal Ligand Exchange with 1,2,3,4-thiatriazole-5-thiolate and Its Facile in Situ Conversion to Thiocyanate: Supplementary Information." The Royal Society of Chemistry, Electronic Supplementary Material (ESI) for Dalton Transactions (2012): n. pag. Print.
- Etgar, L. "Semiconductor Nanocrystals as Light Harvesters in Solar Cells", Materials, p. 445-455, 2013.
- Luber, Erik., Mobarok, M., and Buriak, J. "Solution-Processed Zinc Phosphide (α- Zn3P2) Colliodal Semiconducting Nanocrystals for Thin Film Photovoltaic Applications ", National Institute for Nanotechnology, p.A-K. www.ascnano.org. 2013.
- Yang, Y. N.d. 0. n.p. "Best Research-Cell Efficiencies", National Center for Photovoltaics, https://www.nrel.gov/ncpv/images/efficiency_chart.jpg. 2013. Retrieved October 11, 2013.
- Nikolenko, L. M., and V. F. Razumov. "Colloidal Quantum Dots in Solar Cells", Russian Chemical Reviews 82.5 (2013): 429-48. Print.
- Dharma, Jayant and Pisal, Aniruddha. "Simple Method for measuring the Band Gap Energy value for TiO2 in the Powder Form using UV/Vis/NR Spectrometer". 2012.
- UNICEF, "Progress on Drinking Water and Sanitation 2012: Update", UNICEF and World Health Organization
- Vijayalakshmi, R., and V. Rajendran. "Synthesis and Characterization of Nano-TiO2 via Different Methods." Archive of Applied Science Research 4.2 (2012): 1183-190. Web.
- Hillhouse, Hugh., and Beard, Matthew. "Solar cells from colloidal nanocrystals: Fundamentals, materials, devices, and economics", Elsevier 14 (2009):245-249, 20013. Web.
- Baskoutas, Sotirois, and Adreas F. Terzis. "Size-dependent Bad Gap of Colloidal Quantum Dots." Journal of Applied Physics 99 (2006): n. pag. Web.
- Rogach, Andrey L., Kornowski Andreas, Gao, Mingyuan, Eychmuller, Alexander, and Weller, Horst. "Synthesis and Characterization of a Size Series of Extremely Small Thiol-Stabilized CdSe Nanocrystals." 31December1998
Contact details[edit | edit source]
Alex Poznak APoznak@mtu.edu
Bill Price wjprice@mtu.edu