Background[edit | edit source]
The ability to measure water quality is vital for communities and labs. This can be expensive and inaccessible for many. The purpose of this literature review is to gather the information needed to make an open-source water tester.
The most important pollutants to test for are: chlorine, pathogens, lead,
Literature[edit | edit source]
Water Pollution: Everything You Need to Know[edit | edit source]
M. Denchak, "Water Pollution: Everything You Need to Know," NRDC, May 14, 2018. [Online]. Available: https://www.nrdc.org/stories/water-pollution-everything-you-need-know
Types of Water Pollution:
- Groundwater
- polluted from pesticides, fertilizers, and waste
- for some areas this is the only source of freshwater
- Surface water
- nutrient pollution: nitrates, phosphates
- municipal/industrial waste discharge: toxins
- Ocean water
- chemicals, nutrients, and heavy metals
- oil spills
- Point source
- when contamination originates from a single source
- wastewater discharged from a manufacturer, oil refinery, wastewater treatment facility,
- contamination from septic systems, chemical/oil spills
- originates from a single source, but affects waterways miles away
- Nonpoint source
- contamination derived from several sources
- agriculture/stormwater runoff, debris
- leading cause of water pollution
- difficult to regulate (no one source)
- Transboundary
- contaminated water from one country spilling into the waters of another
Most Common Types of Water Contamination
- Agricultural
- largest consumer of freshwater
- leading cause of water degredation
- rain washes nutrients and pathogens from fertilizers, pesticides, and animal waste into water
- nutrient pollution:excess nitrogen and phosphorous; can cause algae blooms (toxic) that is harmful to people and wildlife
- Sewage and wastewater
- metals, solvents, toxic sludge
- stormwater runoff: rain carries road salts, oil, grease, chemical, and debris into water
- 80% of the world's wastewater flows back into the environment without being treated
- Oil pollution
- 1 million tons of oil pollutes marine environments each year
- 50% is from land sources (farms/cities)
- 10% is from tanker spills at sea
- 30% is from regular operations of the shipping industry
- 1 million tons of oil pollutes marine environments each year
- Radioactive substances
- radioactive waste: any pollution that emits more radiation than what is naturally released by the environment
- generated by uranium mining, nuclear power plants, production/testing of military weapons, and medical research
- can persist in the environment for thousands of years
Water Quality Parameters[edit | edit source]
N. H. Omer, "Water Quality Parameters," Water Quality - Science, Assessments and Policy, Oct. 2019, doi: 10.5772/intechopen.89657. [Online]. Available: https://www.intechopen.com/online-first/water-quality-parameters
Abstract: Since the industrial revolution in the late eighteenth century, the world has discovered new sources of pollution nearly every day. So, air and water can potentially become polluted everywhere. Little is known about changes in pollution rates. The increase in water-related diseases provides a real assessment of the degree of pollution in the environment. This chapter summarizes water quality parameters from an ecological perspective not only for humans but also for other living things. According to its quality, water can be classified into four types. Those four water quality types are discussed through an extensive review of their important common attributes including physical, chemical, and biological parameters. These water quality parameters are reviewed in terms of definition, sources, impacts, effects, and measuring methods.
Classifications of water
- Potable water: safe to drink and usable for domestic purposes
- Palatable water: safe to drink, pleasant to drink, considers the presence of chemicals that do not cause a threat to human health
- Contaminated/Polluted water: water that contains unwanted physical, chemical, biological, or radiological substances. Is unfit for drinking or domestic use
- Infected water: contaminated with pathogenic organism
- Note: domestic purposes are defined as bathing, washing clothes, cleaning etc.
Physical parameters of water quality
- Turbidity
- defined as the cloudiness of water
- a measure of the ability of light to pass through water
- caused by particulates such as clay, slit, and organic materials
- suspended materials can protect micro-organisms from disinfection
- suspended materials can absorb heavy metals and organic pollutants
- higher turbidity raises water temperatures (suspended particles absorb more heat) which decreases the concentration of dissolved oxygen (food for aquatic life)
- measured by a nephelometric turbidimeter in terms of NTU (equivalent to 1 mg/L of silica in suspension)
- Temperature
- palatability, viscosity, solubility, odours, and chemical reactions are influenced by temperature
- affects the biosorption process of dissolved heavy metals
- water is most palatable between 10-15 °C
- Colour
- materials decayed from organic matter (mostly vegetation) and inorganic matter (soil, rocks) colour water
- only for bad for aesthetic reasons not for health reasons
- colour is measured by comparing the sample to standard solutions
- apparent colour: considers the entire water sample, including dissolved and suspended materials
- true colour: considers the water after filtering to remove all suspended material
- graded on a scale of 0 (clear) to 70 colour units
- Taste and odour
- caused by organic and inorganic materials
- can come from natural, urban, or agricultural sources
- numerical value is determined by measuring a volume of sample A and diluting it with B, and odour-free distilled water until the odour of the resulting mixture is just detectable at a total volume of 200 ml
- expressed in terms of a threshold number:
- TON or TTN = (A + B)/A
- Solids
- can occur in solution or in suspension
- can be identified by using a glass fibre filter
- suspended solids are retained on top of the filter and the dissolved solids pass through
- if the filtered water is placed in a dish and then evaporated the dissolved solids remain as a residue
- Total solid (TS) = Total dissolved solid (TDS) + Total suspended solid (TSS)
- freshwater: < 1500 mg/L TDS
- saline water: >5000 mg/L TDS
- Electrical conductivity
- measure of the ability of a solution to carry or conduct an electrical current
- conductivity increases as the concentration of ions in the solution increases
- main parameter used to determine the suitability of water for irrigation and firefighting
- drinking water: 0.005 - 0.05 S/m
- seawater: 5 S/m
Chemical parameters of water quality
- pH
- indicated the strength of an acidic or basic solution
- safe ranges of pH for drinking water are from 6.5 to 8.5
- Note: pH is a logarithmic scale so a change of 1 on the ph scale is a change of 10x of acidity
- a high pH makes the taste bitter and decreases effectiveness for chlorine disinfection
- low pH water will corrode or dissolve metals
- pollution can modify the pH
- a pH bellow 4 or above 10 will kill most fish
- heavy metals dissolve more easily in highly acidic water
- dissolving heavy metals in water can make them more toxic
- a pH change can change the form of some chemicals
- ie: ammonia in basic water becomes more poisonous
- Chloride
- occurs naturally in groundwater, streams, and lakes
- high chloride concentrations in freshwater may indicate wastewater pollution
- chloride ions (Cl-) in drinking water do not cause harmful effects on health, but can cause a salty taste
- NaCl can cause kidney and heart disease, liked to Na
- NaCl causes a salty taste at 250 mg/L
- MgCl and CaCl causes a salty taste at 1000 mg/L
- standard for drinking water is that chloride levels do not exceed 250 mg/L
- can be measured by silver nitrate titration
- Chlorine Residual
- Cl2 does not occur naturally in water, but is added for disinfection
- toxic as a gas, but in dilute aqueous solutions it is not harmful
- the residual concentration ensures good sanitary quality of water
- Chlorine can react with organics in water to form toxic compounds called trihalomethanes (THMs)
- ie; CHCl3 (chloroform) which is carcinogenic
- measured by a colour comparator test kit or spectrophotometer
- Sulfate
- Sulfate ions (SO42-) occur in water
- a high concentration is usually caused by leaching of natural deposits of sodium sulfate (Glauber's salt) or magnesium sulfate (Epson salt)
- high concentrations in drinking water can cause an unpleasant taste or laxative effects but no significant health risk
- Nitrogen
- four forms in water: organic nitrogen, ammonia nitrogen, nitrite nitrogen, and nitrate nitrogen
- if water is contaminated with sewage most of the nitrogen is in organic and ammonia forms
- can be transformed to nitrates and nitrites by microbes
- nitrate is a basic nutrient to the growth of plants
- a high concentration of nitrate in surface water can cause rabid algae growth and degrade the water quality
- nitrates can enter groundwater from fertilizers
- excessive nitrate concentration (>10 mg/L) in drinking water can cause immediate and severe health risk to infants
- nitrate ions react with hemoglobin and reduces the blood's ability to hold oxygen
- Fluoride
- a moderate amount of fluoride ions (F-) in drinking water is good for dental health
- excessive amounts can cause dental fluorosis
- maximum allowable levels depend on local climate
- in warmer regions the max is 1.4 mg/L and in colder regions it is 2.4 mg/L
- 4 methods to determine fluoride ion concentration in water, method chosen depends on the type of water sample
- Iron and manganese
- Fe and Mn do not cause health problems, but do cause a bitter taste even at very low concentrations
- usually occur in groundwater as ferrous (Fe2+) and manganous (Mn2+) ions
- when exposed to air they form insoluble ferric (Fe3+) and manganic (Mn3+)form making water turbid
- can cause black or brown stains on laundry
- can be measured by atomic absorption spectrometry, flame atomic absorption spectrometry, cold vapour atomic absorption spectrometry, electrothermal atomic absorption spectrometry, and inductively coupled plasma
- Copper and zinc
- Cu and Zn are nontoxic in small concentrations
- essential and beneficial for human health and plant/animal growth
- can cause a bad taste in drinking water
- at high concentrations Zn will make water appear cloudy
- can be measure by the same methods as iron and magnanese
- Hardness
- used to describe the properties of highly mineralized waters
- dissolved minerals can cause scale deposits and make lathering soap difficult
- calcium (Ca2+) and magnesium (Mg2+) ions cause the greatest portion of hardness in natural water
- can enter through contact with coil and rock (particularly limestone deposits)
- these ions are present as bicarbonates, sulfates, and sometimes as chlorides and nitrates
- groundwater is generally harder than surface water
- Temporary hardness: due to carbonates and bicarbonates which can be removed by boiling
- Permanent hardness: caused by sulfates and chlorides which remain after boiling
- water with >300 mg/L of hardness is considered hard
- water with <75 mg/L of hardness is considered soft
- hardness up to 500 mg/L is safe by more than that can cause a laxative effect
- can be detected by titration with ethylene diamine tetra acidic acid (EDTA) and Eriochrome Black and Blue indicators
- expressed in terms of CaCO3 mg/L
- Dissolved oxygen
- Chemical oxygen demand (COD) is a parameter that measures all organics with biodegradable and non biodegradable
- it is the amount of oxygen required to chemically oxidize the organic material and inorganic nutrients (ammonia, nitrate) in water
- chemical test using strong oxidizing chemicals (potassium chromate), sulphuric acid, and heat
- COD values are always higher than biological oxygen demand (BOD) values
- Toxic inorganic substances
- even in trace amounts these can be a danger to human health
- some occur from natural sources and other can occur from industrial activities or improper management of hazardous waste
- Metallic compounds: includes some heavy metals that are toxic. Have a wide range of dangerous effects. As and Cr6+ are acute fatal poisons while Cd, Hg, Pb, and Tl cause chronic diseases. Can be measured by atomic absorption photometers, spectrophotometer, or inductively coupled plasma
- Nonmetallic compounds: Includes nitrates and cyanides. Cyanides can cause oxygen deprivation by preventing hemoglobin from carrying oxygen. Can also cause effect on the central nervous system and thyroid. Measured by colorimetric, titrimetric, or electrometric methods
- Toxic organic substances
- man made pollutants such as insecticides, pesticides, solvents, detergents, and disinfectants
- measured by gas chromatographic, high performance liquid chromatographic, and mass spectrophotometric methods
- Radioactive substances
- sources include waste from nuclear power plants, industries, and uranium mining
- when radioactive substances because they release beta, alpha, and gamma radiation
- exposure can cause genetic and somatic damage to living tissue
- radon gas is a great health concern as it occurs naturally in groundwater and is highly volatile
Biological parameters of water quality
- Bacteria
- considered to be single-celled plants
- occur in 3 shapes: bacillus, coccus, and spirellus
- under favourable conditions of food supply, temperature, and pH bacterial can grow to 20 million cells per mm in a day
- many dangerous waterborne diseases are caused by bacteria
- Algae
- support themselves by converting inorganic materials into organic matter
- nuisance organism in water because of their taste and odour
- some species of algae are very dangerous
- blue-green algae can kill cattle and domestic animals
- Viruses
- smallest biological structure that able to reproduce
- parasites that need a host to live
- they can pass through filter that do not permit he passage of bacteria
- waterborne viral pathogens can cause infectious hepatitis
- most waterborne viruses can be deactivated by disinfection
- Protozoa
- single celled microscopic animals
- they can form cysts that are difficult to inactivate by disinfection
- Indicator organisms
- group of bacteria called coliforms which exist in the intestinal system of humans and are expected with body waste
- waste that has recently been contaminated with sewage will always contain coliforms
- most common/well known coliform is E. coli
- aggressive and survive in water longer than most pathogens
- 2 methods to test for coliform bacteria; membrane filter and multiple tube fermentation
Introduction to Atomic Absorption Spectroscopy[edit | edit source]
P. M. V. Raja and A. R. Barron, "1.4: Introduction to Atomic Absorption Spectroscopy," Chemistry LibreTexts, Jul. 13, 2016. [Online]. Available: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_(Barron)/01%3A_Elemental_Analysis/1.04%3A_Introduction_to_Atomic_Absorption_Spectroscopy
Theory:
- atomic absorption spectroscopy (AAS) measures the intensity light absorbed by atomic absorption
- The percentage is compared to a calibration curve to determine the amount of material in the sample
Environmental and marine analysis
- analyses that can be measured very, but include lead, copper, nickel, and mercury
Instrumentation
- Atomizer
- creates a large number of homogenous free atoms
- two types are commonly used: flame and electrothermal
- flame atomizer:
- simple and low cost
- accepts an aerosol from a nebulizer into a flame that can volatile and atomize the sample
- the sample is dried, vaporized, atomized, and ionized
- sub categories of the chemical composition of the flame
- electrothermal atomizer
- graphite tubes increase the temperature
- dries the sample and evaporates the solvent and impurities
- atomizes the sample then raises the temperature to clean the graphite tube
- less harsh than flame atomization
- requires constant temperature, rapid atomization, large solution volume, and minimal radiation emitted
- Radiation source
- irradiates the atomized sample
- the sample absorbs some of the radiation and the rest passes through the spectrophotometer to a detector
- two categories: line sources and continuum sources
- line sources:
- excites the analyte
- emits its own line spectrum
- ex: hollow cathode lamps and electrodes discharge lamps
- continuum sources:
- radiation speeds over a wide range of wavelengths
- typically used for background correction
- ex: deuterium and halogen lamps
- Spectrometer
- separates the different wavelengths of light before they pass to the detector
- can be single or double beam
- single beam:
- only requires radiation that passes directly through the sample
- less optical components
- less radiation loss
- double beam:
- requires two beams of light
- one that passes through the sample and one that does not pass through the sample at all
- more stable over time
- can compensate for changes more readily
Obtaining Measurements
- perfluroalkoxy polymers (PFA), silica, and glassy carbon are often used to store the sample as they will not absorb any trace elements
- acidifying the solution with hydrochloric or nitric acid can help prevent ions from adhering to the walls
- vessels should contain minimal surface area to minimize possible adsorption sites
Calibration Curve
- using a standard, a plot of concentration vs absorbance can be made
- standard calibration technique:
- concentration of the sample is found by comparing its absorbance to a curve of the concentration of the standard vs the absorbances of the standard
- both the standard and the sample must have the same behaviour when atomized
- the error in measuring the absorbance must be smaller than that of sample preparation
- sample must be homogenous
- typically a linear curve using 5 points
- bracketing technique
- variation of the standard method
- two standard with concentrations c1 and c2 bracket the value of the sample concentration
- the sample concentration can be found using the following equation where A is the absorbance
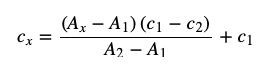
- useful when the concentration of the analyze in the sample is outside the linear portion of the calibration curve
- analyte addition technique
- used when the properties of the sample are expected to create many interferences and the composition is unknown
- instead of using a standard that has a similar matrix to the sample this method uses a portion of the sample itself
- can only be used within the linear range of the absorbances
Analysis
- Precision in the data is 10% or more
- Set 1 produces results within ±5% of the certified values
- Sets 2 and 3 produced results within ±10% of the certified values
- Common issue was that the concentration of an element was at or below the detection limit and could not be analyzed
- S and K had poor detection limits however they are usually in high quantities so it was less of an issue
- Ga, Pt, and Bi are very trace in water and couldn't be detected
Mobile Mass Spectrometer for Determination of Heavy Metals in Sea Water: Numerical Simulation and Experimental Verification[edit | edit source]
V. T. Kogan et al., "Mobile mass spectrometer for determination of heavy metals in sea water: Numerical simulation and experimental verification," Technical Physics, vol. 52, no. 12, pp. 1604–1610, Dec. 2007, doi: 10.1134/s1063784207120134.
Abstract: A prototype mobile mass spectrometer for direct detection of heavy metal traces in water is proposed. The instrument allows automated dosed injection of liquid samples (2.5–20.0 μl) into the lock vacuum chamber of the instrument, extraction of salts containing target components for their following introduction into the high-vacuum chamber of the instrument, and determination of the component concentrations in the initial sample by means of a portable magnetic mass spectrometer equipped with an original electron-impact ion source. Experimental testing of the spectrometer using aqueous solutions containing Zn, Fe, Cu, As, and Cd shows that the instrument can detect heavy metals in water, including salt water, at concentrations ranging from 1 mg/L to 10 μg/L. The experimental results are discussed.
- Dosed water samples are delivered into the mass spectrometer by a jet injection system
- Sample is deprived of water and other volatile substances
- Nonvolatile. compounds (ie: heavy metal salts) are precipitated on the coiled tungsten thread of the extractor
- Up to 10 sample portions can be successively injected before analysis to raise detection sensitivity
- Extractor is a heated substrate used to dry out the sample in vacuum conditions
- Extractor has been integrated into the ion-optical scheme of the ion source
- The source of electrons is a ring filament built into the middle electrode of the ion source
- Conditions for multicomponent express analysis
- target elements enter into one compound dominating the others
- mass spectra of the compounds have characteristic peaks with a fairly high relative intensity
- To reduce formation of complex compounds a pH of 2 should be maintained
- Detection thresholds:
- for Zn, Cu, Fe, and As: 10-100 μg/L
- for Cd: 1 mg/L
A Beginner's Guide to ICP-MS, ICP-MS analysis and basic mass spectrometry[edit | edit source]
"A Beginner's Guide to ICP-MS, Mass Spectrometry basics | Agilent," www.agilent.com. [Online]. Available: https://www.agilent.com/en/support/atomic-spectroscopy/inductively-coupled-plasma-mass-spectrometry-icp-ms/icp-ms-instruments/what-is-icp-ms-icp-ms-faqs
What is ICP-MS
- Elemental analysis technique
- Uses an argon plasma (ICP) to convert the sample into ions that can be measured with a mass spectrometer
- The MS and detector operate in a vacum
- Typically used to analyze liquid samples
- Can measure virtually every naturally occuring element and many "radiogenic" isotopes
- Cannot measure H, He, Ar, N, O, F, Ne
- Can measure elements below 0.1 part pr trillion and up to 1000s parts per million
- covers 10 orders of magnitude
How does an ICP-MS work?
- Sample forms a fine aerosol mist
- ICP converts the elements to ions
- Ions are extracted into the vacuum system
- Ion lens focuses the ions and separates them from background signal
- Collision/reaction cell resolves the analyte ions from interfering ions
- Mass spectrometer filters ions by mass
- Electron multiplier detector
- Data processing
What is ICP-MS used for?
- Liquid sample analysis
- liquid calibration standards are widely available
- total concentrations can be measured against a calibration
- Speciation analysis
- involves coupling the ICP-MS to a form of chromatography device to separate the different chemical forms of an element
- High performance liquid chromatography (HPLC) is the most widely used technique
Quantitative drinking water arsenic concentrations in field environments using mobile phone photometry of field kits[edit | edit source]
E. Haque et al., "Quantitative drinking water arsenic concentrations in field environments using mobile phone photometry of field kits," Science of The Total Environment, vol. 618, pp. 579–585, Mar. 2018, doi: 10.1016/j.scitotenv.2016.12.123.
Digital image capturing and processing
- Field photos taken with a Samsung S Duos-2
- resolution of 5 megapixels
- yielded photos of 1.3 MB JPEG files
- Laboratory photos taken with an iPhone 5S
- resolution of 8 megapixels
- yielded photos pf 2.7 MB JPEG files
- Used the EconoQuick test kit for Arsenic analysis
- Photos of the sample test strip, an unused test strip, and the EQ arsenic test kit concentration colour chart were taken outdoors with indirect sunlight from 60cm away at an angle of 45°
- Digital image processing was conducted using Adobe Photoshop CS6
- photos were normalized using the levels adjustment tool
- goal was to minimize the effect of lighting conditions
- ColorMeter app can be called within a surveying app such as SurveyCTO to analyze the results in real time
- obtains the colour at two spots on the test strips and converts the reading to an estimated concentration of Arsenic
Results
- Agreement between As concentrations predicted with the test kit photo colour data and ICP-MS measured concentrations for field samples
- Accurately quantified concentrations as low as 10 µg/L
Recommended advanced techniques for waterborne pathogen detection in developing countries[edit | edit source]
F. S. Alhamlan, A. A. Al-Qahtani, and M. N. A. Al-Ahdal, "Recommended advanced techniques for waterborne pathogen detection in developing countries," The Journal of Infection in Developing Countries, vol. 9, no. 02, pp. 128–135, Feb. 2015, doi: 10.3855/jidc.6101.
Polymerase chain reaction (PCR)
- Has the ability to amplify a small amount of DNA to millions of copies in less than 4 hours
- Most widely used molecular technique in the the detection of microorganisms in water samples
- Multiplex PCR is a type of PCR where multiple primer sets are used to amplify several targets
- save time and effort
- restricted to a certain number of organics that do not cross-react
- different multiples PCR assays have been developed to detect bacterial pathogens
- Real time PCR is another approach to amplify, quantify, and detect organisms
16S rRNA
- This technique is based on the RNA of the small ribosomal subunit which is universal and abundant
- Can detect the microbe, identify the microbial species, present the sample diversity, and provide a genetic library for microbial population
- Starts with the extraction of nucleic acids from a water sample
- PCR amplification using 16S rRNA universal primers
- Then cloning, sequencing, and identification can occur
- Cannot be used for viruses as they do not contain a diagnostic shared genetic marker
Fluorescent in situ hybridization (FISH) and confocal microscopy
- Useful for determining the abundance of respective populations in a ny given microbial sample
- It enumerates microbial cells, allowing the production of quantitative data of the microbial population
- Technique is based on treating microbial cells with a fixative solution, hybridizing the cells on a glass slide with specific probes, and the visualizing the probes with epifluorescence or confocal laser microscopy
- The combination of FISH and confocal microscopy has become an acceptable monitoring practice in wastewater treatment plants
Microarray
- Based on the hybridization of a target sequence of nucleic acids (RNA or DNA) to a complementary sequence
- Generally target taxonomic genes such as 16S or functional genes
- Electrochemical detection (ECD) 12K microarray
- uses a semiconductor matrix that contains more than 12,000 nucleic acid probes individually synthesized on a single chip
- Main principle is the redox active reaction that allows detection of positively hybridized probes
- successfully used to simultaneously, rapidly, and cost-effectively screen for thousands of pathogens present in a water sample
Mobile Water Kit (MWK): a smartphone compatible low-cost water monitoring system for rapid detection of total coliform and E. coli[edit | edit source]
N. S. Kumar Gunda, S. Naicker, S. Shinde, S. Kimbahune, S. Shrivastava, and S. Mitra, "Mobile Water Kit (MWK): a smartphone compatible low-cost water monitoring system for rapid detection of total coliform and E. coli," Analytical Methods, vol. 6, no. 16, p. 6236, Jun. 2014, doi: 10.1039/c4ay01245c.
Abstract: In this work, we have developed and demonstrated a rapid and low-cost water monitoring sensor that can simultaneously detect total coliform and Escherichia coli (E. coli) bacteria in contaminated drinking water samples. The test method, called Mobile Water Kit (MWK), comprises a set of custom chemical reagents that would serve as colorimetric or fluorometric chemosensors, syringe filter units and a smartphone platform that would serve as the detection/analysis system. The MWK provides information about the presence/absence of total coliform and E. coli in water samples. The MWK has preliminarily been tested for its selectivity, sensitivity and accuracy, with samples of known concentrations of bacteria. The MWK has also been tested with contaminated water samples collected during the two field trials conducted in Canada and India, and the obtained results were confirmed with conventional laboratory methods. With this MWK, we were able to detect the total coliform and E. coli bacteria in water samples within 30 min or less, depending on the concentration of the bacteria. For one of the field samples, the MWK was able to detect the total coliform within 35 s, which is faster than any rapid test methods available in the market. This new technology can dramatically improve the response times for the outbreak of water-borne diseases and will help water managers and individuals to assess the quality of water sources.
- Target concentration value for E. coli is 0 Colony Forming Units (CFU) per 100 mL for potable water
Method
- Collect 100 mL of contaminated water and filter it through a 0.45 µm filter
- Chemical reagents are added onto the syringe filter unit
- A change in colour to red of the filter indicated the presence of total coliform and E. coli
- Change in colour is captured using a smartphone
- Images can be analyzed using the mHealth E. coli app
Chemicals
- 6-chloro-3-indolyl-β-D-galactoside (Red-Gal)
- 4-methyllumbelliferyl-β-D-glucuronide (MUG)
- Bacteria protein extraction reagent (B-PER)
- Laurel Tryptose Broth (LTB) with MUG
- N,N-Dimethylformamide (DMF)
- anhydrous Ferric Chloride (FeCl3)
Formulation of Chemical Reagants
- 4 chemical reagents (A, B, C, and D) were formulated
- Reagent A:
- LTB with MUG (35.7 mg) and Red-Gal (0.3 mg) dissolved in deionized (DI) water (1 mL)
- Reagent B:
- B-PER (1mL)
- Reagent C:
- FeCl3 (20 mg) dissolved in DI water (1 mL)
- Reagent D:
- Red-Gal (30 mg) dissolved in DMF (0.5 mL) and DI water (1 mL)
- For a single test 100 µL of Reagent A and 20 µL each of Reagents B, C, and D are required
- Chemical reagents must be maintained at pH 7
- The combination of Red-Gal and MUG is used to detect E. coli pathogens that secrete β-galactosidase and β-glucuronidase enzymes
Components of the Mobile Water Kit (MWK)
- Can simultaneously test 3 water samples
- Consists of a box with 3 components
- Main unit
- allows placement of 3 syringe filter units, 4 chemical reagents, 12 pipettes to dispense the chemicals onto the filter unit
- Bottom reservoir
- stores the filtered water
- Top lid
- covers the box to avoid exposure to environmental contaminants
- Main unit
- Also contains 3 sterile 100 mL/60 mL leur lock syringes and a sterile container to collect water samples
Water Monitoring
- The mHealth App analyzes the image for the specific colour of red and cancels any background colour
- Can determine the risk level for drinking water sources
Results
- Was able to detect up to 2 CFU per 100 mL with a detection time of 30 to 65 minutes
- For samples with 2 x 108 CFU per 100 mL and 2 x 107 CFU per 100 mL the detection time was 30 to 60 seconds
Development of Cellulosic Paper-Based Test Strips for Mercury(II) Determination in Aqueous Solution[edit | edit source]
S. Wang et al., "Development of Cellulosic Paper-Based Test Strips for Mercury(II) Determination in Aqueous Solution," Journal of Analytical Methods in Chemistry, vol. 2018, pp. 1–7, Nov. 2018, doi: 10.1155/2018/3594020.
- Method is to load a dithizone-containing solution onto paper
- dithizone: C13H12N4S, can produce coloured complexes between the colour-forming agent in the paper strip and Hg2+
- Paper used was medium porosity filter paper
- Dithizone was dissolved in trichloromethane (CHCl3) with a concentration of 0.0127 g/L
- Filter paper was immersed in the solution for 2 minutes at room temperature, and then was dried at 50 °C in a nitrogen protected environment
- The dithizone solution has a 0.33 mg/cm2 loading amount
Results
- Colour absorbance increased with increased time that the test strip was held in the solution to be tested
- Dropping method is more accurate; a certain amount of the solution to be tested is dropped onto the test strip
- The mercury-dithizone complexes were shown to diffuse in indefinite directions
- due to liquid diffusivity
- makes it difficult to determine the colour formed because of the non-uniformity
- was overcame by using a marker to make a 9mm diameter circle on the test strip which served as a hydrophobic barrier to contain the solution
- Final procedure:
- drop 3.7 µL of the mercury solution via pipette onto the circled test strip
- wait 2.5 min for colour to form
- Mercury determination was in the range of 0.1 µg/mL to 30 µg/mL
Development of Cellulosic Paper-based Test Strips for Cr(IV) Determination[edit | edit source]
F. Kong and Y. Ni, "DEVELOPMENT OF CELLULOSIC PAPER-BASED TEST STRIPS FOR Cr(VI) DETERMINATION," BioResources, vol. 4, no. 3, pp. 1088–1097, 2022, Accessed: Jun. 14, 2022. [Online]. Available: https://ojs.cnr.ncsu.edu/index.php/BioRes/article/view/BioRes_04_3_1088_Kong_Ni_Devel_Paper_Test_Strips_Cr6/408
- diphenylcarbazide (DPC) was the colour-forming agent used
- Medium porosity filter paper
- DPC and tricaprylmethylammonium chloride (ammonium salt), 0.2% and 1% respectively, were mixed in acetone
- The filter paper was immersed in this solution for 5 minutes at room temperature
- The paper was dried in air
- After the test, the strip was held in air for 10 minutes for the most accurate colour
- An issue was that the colour developed on the paper was leaching into the solution (40%)
- ammonia salt was added as a hydrophobic species
- far less leaching of colour into the solution
- Absorbance has a linear relationship with the log of [HCrO4-] in the concentration range 0.38x10-5 mol/L - 40.4x10-5 mol/L
References[edit | edit source]
[1] M. Denchak, "Water Pollution: Everything You Need to Know," NRDC, May 14, 2018. [Online]. Available: https://www.nrdc.org/stories/water-pollution-everything-you-need-know
[2] N. H. Omer, "Water Quality Parameters," Water Quality - Science, Assessments and Policy, Oct. 2019, doi: 10.5772/intechopen.89657. [Online]. Available: https://www.intechopen.com/online-first/water-quality-parameters
[3] P. M. V. Raja and A. R. Barron, "1.4: Introduction to Atomic Absorption Spectroscopy," Chemistry LibreTexts, Jul. 13, 2016. [Online]. Available: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_(Barron)/01%3A_Elemental_Analysis/1.04%3A_Introduction_to_Atomic_Absorption_Spectroscopy
[4] V. T. Kogan et al., "Mobile mass spectrometer for determination of heavy metals in sea water: Numerical simulation and experimental verification," Technical Physics, vol. 52, no. 12, pp. 1604–1610, Dec. 2007, doi: 10.1134/s1063784207120134.
[5] "A Beginner's Guide to ICP-MS, Mass Spectrometry basics | Agilent," www.agilent.com. [Online]. Available: https://www.agilent.com/en/support/atomic-spectroscopy/inductively-coupled-plasma-mass-spectrometry-icp-ms/icp-ms-instruments/what-is-icp-ms-icp-ms-faqs
[6] E. Haque et al., "Quantitative drinking water arsenic concentrations in field environments using mobile phone photometry of field kits," Science of The Total Environment, vol. 618, pp. 579–585, Mar. 2018, doi: 10.1016/j.scitotenv.2016.12.123.
[7] F. S. Alhamlan, A. A. Al-Qahtani, and M. N. A. Al-Ahdal, "Recommended advanced techniques for waterborne pathogen detection in developing countries," The Journal of Infection in Developing Countries, vol. 9, no. 02, pp. 128–135, Feb. 2015, doi: 10.3855/jidc.6101.
[8] N. S. Kumar Gunda, S. Naicker, S. Shinde, S. Kimbahune, S. Shrivastava, and S. Mitra, "Mobile Water Kit (MWK): a smartphone compatible low-cost water monitoring system for rapid detection of total coliform and E. coli," Analytical Methods, vol. 6, no. 16, p. 6236, Jun. 2014, doi: 10.1039/c4ay01245c.
[9] S. Wang et al., "Development of Cellulosic Paper-Based Test Strips for Mercury(II) Determination in Aqueous Solution," Journal of Analytical Methods in Chemistry, vol. 2018, pp. 1–7, Nov. 2018, doi: 10.1155/2018/3594020.
[10] F. Kong and Y. Ni, "DEVELOPMENT OF CELLULOSIC PAPER-BASED TEST STRIPS FOR Cr(VI) DETERMINATION," BioResources, vol. 4, no. 3, pp. 1088–1097, 2022, Accessed: Jun. 14, 2022. [Online]. Available: https://ojs.cnr.ncsu.edu/index.php/BioRes/article/view/BioRes_04_3_1088_Kong_Ni_Devel_Paper_Test_Strips_Cr6/408
