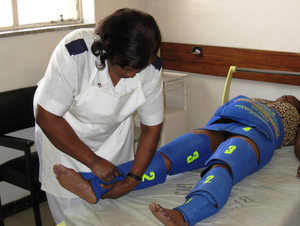
Problem being addressed
The leading cause of maternal mortality is postpartum hemorrhage (PPH). PPH can result from a number of conditions, including uterine atoney and cervical lacerations, which can cause severe blood loss, shock, and then death. Shock can occur due to a lack of oxygen in the heart, lungs, and brain due to a low blood flow in these areas.
Detailed description of the solution[edit | edit source]
The anti-shock garment applies pressure to the legs in order to push blood out of the legs and back into the upper body. Other devices rely on pumps or bladders to dynamically change the pressure applied to the lower body. Instead, the suit makes use of neoprene's elasticity to apply pressure to the legs, which is held in place by velcro straps. The device is washable and reusable, with a lifetime of up to 50 uses.
Designed by[edit | edit source]
The original design was by Zoex. The device was then off patent and a number of well known global health entities funded further clinical trials and mass production of the NASG at a lower price. The funding was provided by The Macarthur Foundation and implemented by PATH. The NASG is now branded LifeWrap by one group and is produced in China and India. As it evolves, the material has been upgraded and now the NASG used widely in global health and low resource settings is made of a different and upgraded material.
Clinical Trials: Several clinical trials in Egypt, Nigeria, India and Pakistan were conducted to evaluate the effectiveness of this device. Dr. Suellen Miller for Life-Wrap (UCSF Dept. of Obstetrics and Gynecology and Reproductive Sciences and the Bixby Centre for Global Reproductive Health) has overseen these trials.
When and where it was tested/implemented[edit | edit source]
The first clinical trials were performed in Nigeria and India. Since then, more trials have been performed throughout Africa and Asia.
Funding Source[edit | edit source]
The MacArthur Foundation, the National Institutes for Child Health and Development, and the Gates Foundation.
References[edit | edit source]
Peer-reviewed publication[edit | edit source]
Turan J, Ojengbede O, Fathalla M, Mourad-Youssif M, Morhason-Bello IO, Nsima D, Morris J, Butrick E, Martin H, Camlin C, Miller S. Positive effects of the non-pneumatic anti-shock garment on delays in accessing care for postpartum and postabortion hemorrhage in Egypt and Nigeria. J Womens Health (Larchmt). 2011 Jan;20(1):91-8.
Berdichevsky K, Tucker C, Martínez A, Miller S. Acceptance of a new technology for management of obstetric hemorrhage: a qualitative study from rural Mexico. Health Care Women Int. 2010 May;31(5):444-57.
Miller S, Lester F, Hensleigh P. Prevention and treatment of postpartum hemorrhage: new advances for low-resource settings. J Midwifery Womens Health. 2004 Jul-Aug;49(4):283-92. Review.
Other internally generated reports[edit | edit source]
MacArthur Foundation. (2007). Using the Anti-Shock Garment to Save Lives. Retrieved November 13, 2011 from http://www.macfound.org/site/c.lkLXJ8MQKrH/b.2351709/apps/nl/content2.asp?content_id=%7BC411C1FD-A7D5-40E7-9759-B9CA21C3E27D%7D¬oc=1.
Wilder, Jennifer. (2011). "Low Tech Saves Lives." Global Health Magazine. Global Health Council. Retrieved November 13, 2011 from here.
Externally generated reports[edit | edit source]
BBC News. (Feb 28 2006). "Birthing Suit 'could save Lives'" Retrieved November 13, 2011 from http://news.bbc.co.uk/2/hi/health/4757978.stm
New York Times, Health Section. Small fixes: Low cost innovations that can save thousands of lives. Like a Wetsuit, but It can Save a Hemorrhaging Mother http://www.nytimes.com/2011/09/27/health/27wetsuit.html?_r=2&ref=health
IP and copyright[edit | edit source]
US Patent Number 3933150 - Medical pneumatic trouser for emergency autotransfusion.
Approval by regulatory bodies or standards boards[edit | edit source]
510(k) FDA approval (http://maternova.net/category/innovation-purpose/medical-devices?page=6)
The NASG is approved by the WHO.