No edit summary |
No edit summary |
||
| Line 70: | Line 70: | ||
===Etched Surface Si-C Anodes=== | ===Etched Surface Si-C Anodes=== | ||
====Method==== | |||
The problem of facturing in silicon causes a loss of contact with the current collector. To help the preformance of silicon carbon anodes is to etch the surface of the current collector. With a etched surface the Si-C material has more surface area to bond to and resists delaimanting from the surface of the current collector.<ref name="[10]">10 Kim Y. L, Sun Y. K, Lee S. M. (2008). Enhanced electrochemical performance of silicon-based anode material by using current collector with modified surface morphology. Electrochimica Acta, 53, 4500-4504.</ref> | |||
===Silicon Carbon Composites=== | ===Silicon Carbon Composites=== | ||
Revision as of 00:24, 2 December 2009
Introduction
With increasing amount of mobile and high energy demand technology there is a need for high density, low weight and small size energy storage system. This was the drive to produce Lithium ion batteries in the 1970’s during the oil crisis [1]. This led to the creation of Lithium ion batteries using lithium metal as an anode. This was done because of Lithium’s properties being ideal for this use. The problem was that during charging and discharge cycles was that dendrites would grow on the surface of the anode and eventually contact the cathode causing a short circuit which would heat the battery and cause it to overheat which lead to explosions and fire [1]. The break though came in the early 90’s when the first lithium ion batteries that used a carbon anode that lithium could diffuse into which eliminated dendrite growth and made the batteries safe to use [1]. Now driving the development of higher energy density batteries are the abundance of energy hungry mobile devices and energy storage for houses and electric vehicles that cannot have massive batteries attached to them.
Existing Technology
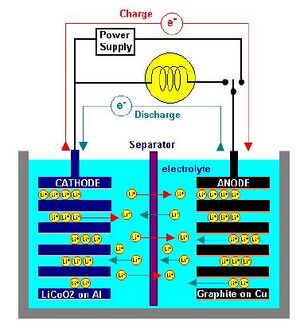
The existing lithium ion batteries are mostly based on a carbon anode with a LixO alloy cathode [2]. This technology is very similar to the original technology when lithium ion batteries were introduced. There have been some improvements to the batteries such as surface treatments but none have really dramatically increased the energy density of the batteries which is around 372mAh/g [1]. A lithium ion battery works by exchanging Li ions between the anode to cathode during discharge and the reverse during charging the reaction is as follows [1].
6C + LiCoO2 ↔ Li1-xCoO2 + LixC6 [1]
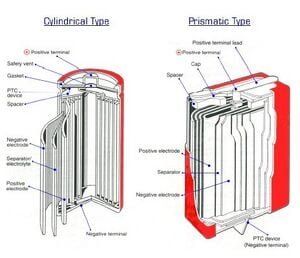
The two types of cell construction are cylindrical cells and prismatic cells. This is only the shape of the cell the internal components are exactly the same [2].
The internal components consist of a cathode that is usually made of aluminum as the current collector with a coating of the lithium cobalt oxide. The anode is traditionally made out of carbon usually in the form of graphite which is on a copper current collector [2]. The anode and cathode are separated by a separator that is made out of polyethylene or polypropylene film that is porous to allow for particle diffusion [2]. The electrolyte used is a organic solvent. All of these components are arranged inside of the cell casing made of steel or aluminum [2]. There are multipliable layers of anodes and cathodes in each cell.
Properties of Silicon
Advantages
Silicon is being looked at as a material to replace carbon as anodes in lithium ion batteries because of the potential of vastly increasing the specific capacity that the batteries could archive. Silicon has the highest theoretical specific capacity of all metals at 4200mAh/g [3]. This is compared to regular carbon anodes with a specific capacity of carbon which is 372mAh/g [1].
Problems
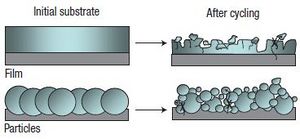
The largest problem to overcome when using silicon is the strains that are associated with the huge volume expansion that occurs during the lithiation of the silicon. The silicon can increase in volume by up to 400%[5]. The volume change causes fracturing of the lithium and after several cycles the lithium losses electrical contact with the current collector [4].
Types of anodes
There are several approaches used to overcome the problem of the loss of electrical contact due to volume expansion. There are several different methods currently being study to allow the use of silicon in lithium ion batteries. Regardless of method all of the approaches aim to maintain capacity during cycling well increasing the specific capacity of the battery.
Amorphous Film Silicon
Method
To counter the problem of fracturing of the anode amorphous silicon could be used. Amorphous silicon has shown that it remains stable even after repeated lithium ion insertion.[6] Using a pure silicon in a bulk form has the advantage of having a larger amount of silicon per unit volume.
Manufacture
There are several methods to create amorphous silicon. A common method is to use DC Magnatron Sputtering. This process is a phsyical vapour deposition process used to deposit flims on to substrats. During the process a target is bombarded by energenic ions which dislodge atoms on the target material. Under vacumn pressures the atoms then fly around unit they stick to a sutable substrate. The atoms that are dislodge are usally ions so they can be diercted with voltage onto the subsrate.
Advantages
The amorphous silicon anodes show a very good charge capacity of 3000mAh/g. This is about 10 times larger than that of current carbon anodes. The amorphous silicon also out proforms bulk silicon during repeted charge and discharge cycles with the capacity with minamial fading out to a large number of cycles.[6]
Etched Surface Si-C Anodes
Method
The problem of facturing in silicon causes a loss of contact with the current collector. To help the preformance of silicon carbon anodes is to etch the surface of the current collector. With a etched surface the Si-C material has more surface area to bond to and resists delaimanting from the surface of the current collector.[7]
Silicon Carbon Composites
Method
One way of improving the cycle performance of silicon is to reduce the size of the particles that are used in the anode and coat them in
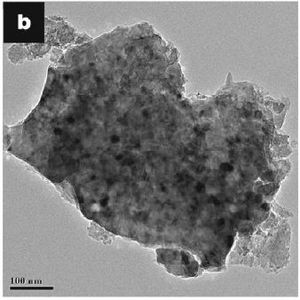
[8] carbon. This size reduction along with the carbon cause smaller stresses in the silicon and the coating of carbon help to maintain the electrical contact with the current collector [9].
Manufacture
The Si-C composite can be made using thermal vapour deposition (TVD) or carbon netting of silicon [8]. With each process the starting material is silicon less then 100nm in diameter. These fine particles are achieved though mechanical milling. During the TVD process benzene or toluene and silicon is heated up to 1000 ⁰C the vapours of the coating collects on the silicon and decomposition of the benzene or toluene occurs on the surface of the silicon [8]. The carbon netting method is slightly more involved. The silicon is first dispersed in a solution of sucrose with sulphuric acid being added to it. The solution is then dehydrated well it is being mechanically agitated [8]. Regardless of preparationmethod the carbon contains the silicon and keeps the integrity of the active particles [8].
Advantages
The Si-C composites outperform pure silicon during cycling by maintaining a higher specific charge even when the specific charge of pure silicon drops off drastically. This is due to the carbon controlling the volume change and its electrical conductivity helping to keep the silicon in electrical contact with the current collectors [8].
Silicon Nanowires
Method
To also counter the problems caused by the volume changes in silicon is to use nanowires. The wires are directly on the stainless steel
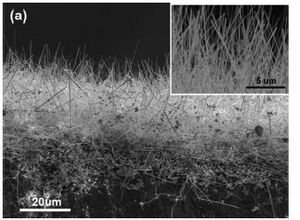
[4] current collector. This means that every wire is connected directly to the current collector and all wires contribute to the capacity. The wires also have more efficient 1D electronic pathways [4].
Manufacture
The nanowires can be grown using VLS (vapour-liquid-solid) deposition process. The substrate is first coated in Au which is the catalyst for the nanowire growth. The substrate is the heated to melting point of the catalyst. An atmosphere of silicon vapour and inert gas is pumped into the chamber and the silicon becomes super saturated in the catalysis and participates out of solution and grows the wire [5].
Advantages
The nanowire anodes show in creditable specific capacity at a first cycle charging specific capacity equal to the theoretical value of 4200mAh/g (5). After the first cycle however the charge specific capacity dropped down to 3541mAh/g this stayed stable during more cycles and little fading happened. This shows that the nanowires are resisting the volume change and staying firmly attached to the current collector and not fracturing into particles [4].
Implementation
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Fu. L. J, Liu. H, Li. C, Wu. Y. P, Rahm. E, Holze. R, Wu. H. Q. (2006). Surface modifications of electrode materials for lithium ion batteries. Solid State Sciences, 8, 113-128
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Lithium Ion Technical Manual. (n. d). Retrieved from http://www.tayloredge.com/reference/Batteries/Li-Ion_TechnicalManual.pdf
- ↑ Xu. Y. H, Yin. G. P, Zuo. P. J. (2008). Geometric and electronic studies of Li15Si4 for silicon anode. Electrochimica Acta, 54, 341-345.
- ↑ 4.0 4.1 4.2 4.3 4.4 Chan C. K, Peng H, Liu G, McIlwarth K, Zhang X. F, Huggins R. A, Cui Y. (2008). High-Performance lithium battery anodes using silicon nanowires. Nature Nanotechnology, 3, 31- 35
- ↑ 5.0 5.1 Ruffo R, Hong S. S, Chan C. K, Huggins R. A, Cui Yi. (2009). Impedance Analysis of Silicon Nanowires Lithium Ion Battery Anodes. J. Phys. Chem, 113, 11390-11398.
- ↑ 6.0 6.1 Baranchugov V, Markevich E, Pollak E, Salitra G, Aurbach D. (2007). Amorphous silicon thin flims as a high capacity anodes for Li-ion batteries in ionic liquid electrolytes. Electrochemistry Communications, 9, 796-800.
- ↑ 10 Kim Y. L, Sun Y. K, Lee S. M. (2008). Enhanced electrochemical performance of silicon-based anode material by using current collector with modified surface morphology. Electrochimica Acta, 53, 4500-4504.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Yang X, Wen Z, Zhu X, Huang S. (2005). Preparation and Electrochemical Properties of Silicon/Carbon Composite Electrodes. Electrochemical and Solid State Letters, 8, A481-A483.
- ↑ Qin S, Hanai K, Imanishi N, Kubo M, Hirano A, Takeda Y, Yamamoto O. (2009). Highly reversible carbon-nano-silicon composite anodes for lithium rechargeable batteries. Journal of Power Sources, 189, 761-765.