No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
[[Gallium arsenide]] (GaAs) is a semiconductor material that is used in a wide variety of applications ranging from circuits to [[solar cells]]. Solar cell of GaAs can be produced using both bulk and thin film growth methods. | [[Gallium arsenide]] (GaAs) is a semiconductor material that is used in a wide variety of applications ranging from circuits to [[solar cells]]. Solar cell of GaAs can be produced using both bulk and thin film growth methods. | ||
==Material Processing== | |||
==Bulk Growth== | [[File:Manufacturing process of GaAs.png|thumb|left|Manufacturing process of GaAs and other semiconductors with the waste products produced]] | ||
===Bulk Growth=== | |||
There are two common ways to produce GaAs using bulk growth, Liquid-Encapsulated Czochralski (LEC) growth, and Vertical Gradient Freeze (VGF) technology. <ref> | There are two common ways to produce GaAs using bulk growth, Liquid-Encapsulated Czochralski (LEC) growth, and Vertical Gradient Freeze (VGF) technology. <ref> | ||
R.L. Adams, Growth of high purity GaAs using low-pressure vapour-phase epitaxy, Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, Volume 395, Issue 1, 1 August 1997, Pages 125-128, ISSN 0168-9002, 10.1016/S0168-9002(97)00624-4. | R.L. Adams, Growth of high purity GaAs using low-pressure vapour-phase epitaxy, Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, Volume 395, Issue 1, 1 August 1997, Pages 125-128, ISSN 0168-9002, 10.1016/S0168-9002(97)00624-4. | ||
| Line 17: | Line 18: | ||
VGF growth works by placing high purity arsenic and gallium in an enclosed quartz ampoule with a crystal of GaAs. The arsenic and gallium are melted, and then brought into contact with the GaAs crystal. When cooled slowly, a single crystal of GaAs is formed. The single crystal formed has many of the same impurities as LEC growth crystals, which restricts the utility of the crystals. | VGF growth works by placing high purity arsenic and gallium in an enclosed quartz ampoule with a crystal of GaAs. The arsenic and gallium are melted, and then brought into contact with the GaAs crystal. When cooled slowly, a single crystal of GaAs is formed. The single crystal formed has many of the same impurities as LEC growth crystals, which restricts the utility of the crystals. | ||
Typical dimensions of the semiconductor crystals are 1-6 inches in diameter and 2-30 inches long. The usual rate of crystal growth is 1-5mm per hour. <ref name="[2]"> Swartzbaugh Joseph, Sturgill Jeffery. Reduction of Arsenic Wastes in the Semiconductor Industry, 1998 </ref> | |||
===Cutting, Polishing, and Etching=== | |||
The bulk crystals (boule) has is generally cylindrical with conical ends, which are usually cut off and wasted because their diameter isn't big enough to be used. Next, the boule is ground to ensure a uniform diameter over the length of the boule which is usually done with a lathe, or similar tool. The crystals need to be aligned using x-ray diffraction and then the boule is cut into wafers. <ref name="[2]"> </ref> | |||
==Thin Film Growth== | ==Thin Film Growth== | ||
Revision as of 21:19, 4 October 2011
Introduction
Gallium arsenide (GaAs) is a semiconductor material that is used in a wide variety of applications ranging from circuits to solar cells. Solar cell of GaAs can be produced using both bulk and thin film growth methods.
Material Processing
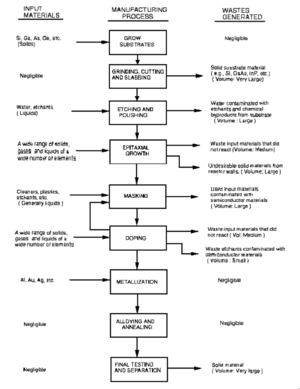
Bulk Growth
There are two common ways to produce GaAs using bulk growth, Liquid-Encapsulated Czochralski (LEC) growth, and Vertical Gradient Freeze (VGF) technology. [1]
LEC growth is accomplished by melting high-purity arsenic and gallium in a high temperature vessel, and slowly cooling to produce a single crystal. The GaAs crystal produced using this method however has some impurities such as significant levels of carbon, and numerous dislocations. These impurities cause the semiconductor to be unusable for some applications.
VGF growth works by placing high purity arsenic and gallium in an enclosed quartz ampoule with a crystal of GaAs. The arsenic and gallium are melted, and then brought into contact with the GaAs crystal. When cooled slowly, a single crystal of GaAs is formed. The single crystal formed has many of the same impurities as LEC growth crystals, which restricts the utility of the crystals.
Typical dimensions of the semiconductor crystals are 1-6 inches in diameter and 2-30 inches long. The usual rate of crystal growth is 1-5mm per hour. [2]
Cutting, Polishing, and Etching
The bulk crystals (boule) has is generally cylindrical with conical ends, which are usually cut off and wasted because their diameter isn't big enough to be used. Next, the boule is ground to ensure a uniform diameter over the length of the boule which is usually done with a lathe, or similar tool. The crystals need to be aligned using x-ray diffraction and then the boule is cut into wafers. [2]
Thin Film Growth
Thin films of GaAs have many advantages over large single crystals of GaAs when it comes to being used in solar cells. Thin films lack some of the impurities found in large crystals, and are capable of being used without requiring extensive slicing. The rest of the case study will be dedicated to thin film GaAs semiconductors.
The most common thin film growth methods for producing GaAs films are Vapour Phase Epitaxy (VPE), Metalorganic Chemical Vapour Deposition (MOCVD), and Molecular Beam Epitaxy (MBE).
Plausibility of Recycling
Amount of material in a typical cell = 3100 g/m2
Peak Power = 272.8 W/m2
Amount of material per Watt Peak = 11.4 g/Wpeak[3]
Metalorganic Chemical Vapour Deposition (MOCVD)
Material utilization efficiency = 30%[4]
Waste material rate MOCVD = 2170 g/m2 and 7.98 g/Wpeak
Molecular Beam Epitaxy
Material utilization efficiency for Ga = 40-70%
Material utilization efficiency for As = 10-20%
Average Material utilization efficiency = 35%
Waste material rate MBE = 2015 g/m2 and 7.41 g/Wpeak
Forms of Collection of Waste
References
- ↑ R.L. Adams, Growth of high purity GaAs using low-pressure vapour-phase epitaxy, Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, Volume 395, Issue 1, 1 August 1997, Pages 125-128, ISSN 0168-9002, 10.1016/S0168-9002(97)00624-4. (http://www.sciencedirect.com/science/article/pii/S0168900297006244) Keywords: Low-pressure vapour-phase epitaxy; LPVPE; GaAs
- ↑ 2.0 2.1 Swartzbaugh Joseph, Sturgill Jeffery. Reduction of Arsenic Wastes in the Semiconductor Industry, 1998
- ↑ http://www.spacequest.com/products/SP-X.pdf
- ↑ http://www.bnl.gov/pv/files/pdf/art_168.pdf