Background
The Systems Engineering Research Center (SERC) is a Department of Defense (DoD) University Affiliated Research Center (UARC) at Stevens Institute of Technology; the Office of the Deputy Assistant Secretary of Defense for System Engineering (ODASD/SE) sponsors SERC activities. SERC is chartered to strengthen academic research in system engineering, addressing problems of interest to DOD. A component of SERC’s effort is the “Capstone Marketplace”, which connects DOD and other government organizations with undergraduate academic teams to work on design projects. The Capstone Marketplace is a resource which provides student design teams research topics, contact with government Subject Matter Experts, and research funding for projects. University teams are expected to operate like small industry teams, performing research and development for government “SME” “customers”. SERC staff will provide technical, business, and other management references and resources as needed. Army units require new, backpack portable fuel and power sources and electrical storage systems that reduce the size, weight, volume and logistics burdens associated with currently available battery systems. Users require high performance battery technologies that increase the specific energy and specific power capabilities of current batteries as well as increasing their recharging performance, depths of discharge, cycle life, and operational robustness. The Blue Marble Security Enterprise (Blue Marble) part of the Enterprise Program which is made up of student run, multidisciplinary, project based, for academic credit, teams who function more like an entrepreneurial start up or small business then a classroom course. Blue Marble is housed within the Department of Electrical and Computer Engineering and technical, professional communication, and project management skills through real world, industry or government sponsored Capstone projects. The Blue Marble team has over 10 years of project work, specifically among student in ROTC or those interested in defense careers. Recent projects had energy storage density aspects to them with innovative solutions provided by the student team. Through the Enterprise Program, students build technical, professional communication, and project management skills through real world, industry or government-sponsored Capstone projects. An opportunity exists for a student team design, test, and prototype battery technologies that increase specific energy, specific power capacities, and increase charging performance, depths of discharge, cycle life and operational robustness. This project will require the student project team to investigate and document shortfalls of the current battery systems, capture and understand all design constraints, develop and justify concepts, and develop a prototype level back-packable power system. A successful project will enhance the education of the undergraduate student team while advancing the Army’s mission of developing a new back-packable power source.
Project
Flow of Development Fall 2018
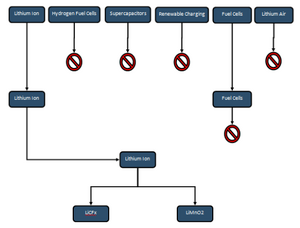
Regarding the flow of the Army Backpack project the team decided to split the work into the two semesters. The Fall 2018 semester would focus on research and determining one or two possible solutions to replace the BA5590, and then the Spring 2019 semester would be focused on testing and prototyping the batteries for use. The Fall 2018 semester began with the entire team meeting for the first time and determining a proper course of action for the project. It was determined that for the first week each member should try to find at least three types of solutions that would improve upon the base values given by the BA5590 datasheet. The team presented several ideas, examples being supercapacitors, fuel cells, lithium ions, hydrogen fuel cells, and renewable charging. From here the team discussed the feasibility of each of the options, and some of the options were ruled out, specifically supercapacitors, hydrogen fuel cells, and renewable charging. The team further did research and narrowed down the choices to two specific types of lithium ion batteries, LiMnO2 and LiCFx. From here the team was split into two pairs, each taking a specific type of battery to further research allowing the team to make a well informed decision for the final battery to be presented to the sponsor. In wanting to make sure both options were tested the team decided to begin building battery packs and determining the types of tests that would need to be done in the following semester to determine the best option to replace the BA5590. An overall flowchart of the teams development over the course of the semester for the battery can be seen below.
Flow of Development-Spring 2019
With the previous semester being used to select the LiMn2O4 battery type, the team sat down together the first week to determine a course of action for the spring semester of the project. As a first step, the team split into two separate subteams in order to research a battery management system for the pack as well as possible directions to take the testing in once the system was created. With these aspect of the project decided on, parts were ordered so that the team would have extra for the pack creation as well as the battery management system that would essentially monitor the battery as it was used. The following weeks were spent in the actual production and set up for the prototype that the team would present to SERC. The team was briefed on the use of a spot welder used for assembly and team members researched the most efficient means of circuiting the battery for best output while conserving space and weight. With the training and design finished the group spot welded the previously ordered LiMn2O4 cells together to assemble the pack. A 3D model of the battery pack case was also designed with an intent from the team to print it as well as an NX model created for the packs housing in the backpack for a conceptual view.
Research
LiMn2O4
One of the solution options that was chosen to be further investigated in the spring semester was LiMn2O4, lithium manganese oxide, battery configuration. Within the team’s research it was determined that SAFT had a prior contract with a battery of the same chemical configuration in 2005 however this variation was non-rechargeable. The Army Backpack team is working with the same chemical make up battery, however striving to reach a rechargeable battery at the end which would improve upon the BA5590 currently used. The team is working on constructing a battery pack to be tested next semester that should be rechargeable. LiMn2O4 appeared to be an effective solution to the problem statement provided by the sponsor for several reasons. Lithium manganese oxide is an already common type of battery that has applications in military as stated prior, and is widely used on the consumer market holding approximately 80% of the market. Upon basic research it showed that LiMn2O4 batteries do have a smaller range of operating temperatures than that of the BA5590 however this can be altered just as its specific energy, lifespan, and specific power can be maximized depending on the application that it is being used in. The three items, specific energy, specific power, and lifespan are also three criteria to be improved upon as stated in the project summary statement. The current operating temperature of the BA5590 is -40℃ to 71℃, while LiMn2O4 has an operating range of -30℃ to 60℃. In regards to the specific energy of the generic LiMn2O4 battery there is a specific energy of 100 - 150 Whr/kg, which in comparison to the BA5590 specific energy at 500 Wh/kg is significantly smaller. However, as stated prior the battery can be altered, due to it being flexible battery, to maximize the specific energy to get it equal to or exceeding the BA5590, LiSO2 battery. The LiMn2O4 research also showed that it has a significantly slower discharge rate allowing it to be used for example with a communication system twice as long as the current BA5590 battery. A chart can be seen in Figure 6 that shows the results described, which is another reason why the team chose LiMn2O4 to be a finalist to replace the LiSO2 battery.
LiCFx
The other finalist was a lithium carbon monofluoride battery. (LiCFx) The specific energy of LiCFx the battery far surpassed the BA5590 that the team is trying to replace. And with an energy density of around 2200 Wh/Kg we knew we’d be able to get that power in a lighter battery or more power in a equally sized battery if required. The operating temperature range of this battery are between -20℃ to 120℃ making it slightly more susceptible to the cold that the BA5590 with its -40℃ to 71℃ range, but given current climates where this battery would be used the heat resistance it offers may be of more importance. Combined these two significant factors along with the large operating range of the battery, the longer discharge time than even the other finalist which is shown in the figure below, and it’s 15 year shelf life this battery type is very promising. A few weak points of the battery we are aware of so far are that most of the market for these batteries is for smaller button type batteries, about 62.3%, and only about 4.3% of the market is prismatic. So getting enough of the batteries to combine to make a big enough ones to rival the current used battery may be a challenge if these were to go into widespread use for the military. Also the cost for these batteries is higher than the other practical options we came across. The battery technology has already been tested in the field as well as the UK has 17 Ah cells used in their version of the BA5590. This battery concept was eventually scrapped due to not being able to find a rechargeable version of it.
Fuel Cell
The fuel cell proposal was very promising early on in our research. The primary cell we looked into was the Alkaline Fuel Cell (AFC) which has been used in various applications over the past 60 years. This type of fuel cell uses the redox reaction of oxygen and hydrogen, producing electrons and water in the process. The primary benefit of the AFC is the relatively specific energy and energy density. In fact, the specific energy can be up to 300 times greater than a lithium-ion battery and have 10 times the energy density. Another attractive feature of the AFC is the operating temperature of up to 90℃. The last benefit from the AFC is that you can use non-noble metals at the anode, which saves in cost and weight. There are a few issues with hydrogen fuel cells as portable power supplies though. Much like batteries, multiple tanks must be stored on the person for longer missions. Also, compressed air requires a relatively study tank which would add more weight to the system. Another issue is that each fuel cell produces only a fraction of a volt, typically in the 0.5-1V range. In order to produce the voltage needed, the system would require a stack of at least 12 cells in series to produce the minimum voltage option of the BA5590. Every individual cell will add to the weight, portability, and cost of the AFC. Although there are some benefits of the fuel cell as a portable system, it is best suited for a non-mobile application.
Lithium Polymer
A significant portion of the way through the spring 2019 semester another student in the Blue Marble Security Enterprise brought up a lithium polymer battery, and how it may be a better option than the LiMn2O4 the team had chosen. One member was assigned with researching the lithium polymer (LiPO) battery to determine if it would be another option. LiPO batteries have a limited use in the public market due to being approximately 30% more expensive than normal lithium ion batteries. As it can be seen in Figure 8 the LiPO battery has a 20% increase on cycles than that established in last semesters research for LiMn2O4.
LiPo batteries also match the specific energy for LiMn2O4 that was determined in the fall with 100-150 Whr/kg and 100-158 Whr/kg for LiMn2O4 and LiPO batteries respectfully. Even with matching the specific energy of LiMn2O4, LiPO batteries as stated prior are not as commercially used thus are more expensive to purchase, the operating temperature range is smaller at -20℃ to 60℃, and finally LiPO can become highly unstable if the proper procedure for charging is not followed. Thus, the option of LiPO batteries comes in second to the already selected LiMn2O4 battery. However if the project is to be extended for another semester a more indepth research will be conducted by the team.
Plan of Selection
When all the options for a solution for battery were presented at the second meeting, the team determined an appropriate way to determine which solutions would be removed from the list. A decision matrix was created which listed all of the options presented, as well as the design characteristics that the team determined were important to compare against the BA5590. The decision matrix can be seen in the figure below.
A few of the options had been crossed out on the decision matrix in Figure 6 due to there not being a significant amount of information present, or it being still in the theoretical stage like the option of lithium air. Several of the characteristics listed at the top came from the project statement document given by the sponsor like recharge rate, and discharge rate. The other characteristics were thought up by the team during a brainstorming session. It was determined that manufacturability was an important characteristic, due to the battery needing to be easily manufacturable to produce the amount needed for the Army.
Battery Management System
In the case of building your own Lithium ion battery it is recommended to have a battery management system to ensure proper operation. The battery management system, bms, simply keeps track of the batteries when they are charging and discharging. Its purpose is to protect the batteries and the user of them. It does this when the battery is in use by monitoring each cells voltage and current from it so no one cell gets too stressed. While charging it does a similar activity but in reverse making sure to charge each cell and preventing overcharging of not just the pack as a whole but of each individual cell within. Since each different battery isn’t chemically identical, close but not perfectly, they will charge at different rates and the bms monitors this and will cut off the cells from being charged as the reach full charge.
NX Case Model
The case model was based on how the battery pack was assembled, the team decided to build a case similar to the one used for the old battery. The new case dimensions are 5.9in x 2.8in x 2.6in, these measurements came from the packed battery dimensions plus battery management system, taking into account that the case would need to have a shell as seen in Figure 10 and some extra space for cable connections. The team decided it would be better if the top of the case could slide off in case repairs needed to be made to the battery pack, so a slot was also added to the final design.
Assembly of Pack
For the assembly of the of the pack the goal was to match or beat the BA5590’s specs. With this in mind we found that our voltage of our batteries was around 3.6 volts per battery. We went with the typical capacity values found on the BA 5590’s spec sheet as a goal to match. This meant we had to get about 9.1Ah at 24V or 18.2Ah at 12V. We opted for the 24 volts and 9Ah since this gave us a good match for the to shoot for while also being roughly the same size as the BA5590 for comparison. In the end, this lead to seven batteries in series and three sets of them in parallel. This gives us about 23.2V and 9Ah, but is also only about 60% the size of the old battery so for something similarly sized we could get more amp hours out of it.
Conclusion
Improvements
The dimensions of the battery pack have been shrunk while still matching the previous battery. The old battery measured 2.45in x 4.4in x 5in or a total of 53.9 cubic inches. Our battery measures 5.7in x 2in x 2.6in for a total of 30.16 cubic inches. So in total our battery takes up 56.01% the space of the older battery. Due to ordering a bulk of batteries before we knew the amount needed we settled at matching old battery, but with the amount of space saved we could easily double the amp hours while barely making the battery any bigger.