ABSTRACT
The rice hulls are unique within nature. They contain approximately 20% opaline silica in combination with a large amount of the phenyl propanoid structural polymer called lignin. This abundant agricultural waste has all of the properties one could ever expect of some of the best insulating materials. Recent ASTM testing conducted R&D Services of Cookville, Tennessee, reveals that rice hulls do not flame or smolder very easily, they are highly resistant to moisture penetration and fungal decomposition, they do not transfer heat very well, they do not smell or emit gases, and they are not corrosive with respect to aluminum, copper or steel. In their raw and unprocessed state, rice hulls constitute a Class A or Class I insulation material, and therefore, they can be used very economically to insulate the wall, floor and roof cavities of a super-insulated Rice Hull House. This paper also explains how the structure of such a house can be fashioned out of a variety of engineered lumber products derived from sugarcane rind.
PAPER
When nature decided how to package a grain of rice, she wrapped this tiny bundle of nutrients with what is often referred to as a “biogenic opal.”[1] The chemical structure of the rice hull, containing amorphous silica bound to water, closely resembles that of the opal, and this gives the rice hull some fairly amazing properties. Nowhere could we ever find a cereal by-product so low in protein and available carbohydrates, and yet, at the same time, so high in crude fiber, crude ash and silica.[2] Of all cereal byproducts, the rice hull has the lowest percentage of total digestible nutrients (less than10%).[3]

The rice hull contains approximately 20% opaline silica in combination with a large amount of the phenyl propanoid structural polymer called lignin. Such a high percentage of silica is very unusual within nature,[4] and this intimate blend of silica and lignin makes the rice hull not only resistant to water penetration and fungal decomposition, but also resistant to the best efforts of man to dispose of it. Since rice is grown on every continent except Antarctica, since it ranks second only to wheat in terms of worldwide area and production,[5] and since the hull represents on average about 20% of the rough harvested weight of rice,[6] our planet ends up with an abundance of this scaly residue.
More than 100,000,000 metric tons of rice hulls are generated each year throughout the world.[7] In 1995, the United States produced about 1,260,000 metric tons of rice hulls[8] at about 50 mills[9] located in Louisiana, Texas, Arkansas, Missouri, Mississippi, Florida and California. Since most mills store rough rice and process it on a daily basis, fresh dry hulls are available throughout the year. Since hulls do not biodegrade or burn very easily, they are sometimes available free-of-charge.
- Husks typically sell for about $6/ton, although one mill indicated that it has sold husks for prices ranging from $2 to $20 per ton.[10]
The hull is a very tough and abrasive packaging material, consisting of two interlocking halves. It encapsulates the tiny space vacated by the milled grain, and in proximity to a myriad of other hulls, it forms a thermal barrier that compares well with that of excellent insulating materials.[11] Thermal resistance tests on whole rice hulls indicate R-values greater than 3.0 per inch.[12] If the R-value of rice hulls is so favorable, why have they not been used extensively to insulate residential and commercial structures?[13]

Perhaps our scientists and engineers focus only on creating materials and products that can be labeled and marketed as proprietary. Perhaps the humble use of the rice hull as an insulation material does not sufficiently inspire the scientific or commercial imagination. But why focus on man-made products when natural materials abound? Surely there must be some profound and obvious reason that makes the raw rice hull unsuitable to serve as an insulation material.
Do rice hulls burn? Yes they do, but with difficulty, as Eldon Beagle once so elegantly explained:
- "The peculiar silica-cellulose ‘drinking-straw bundle’ structural arrangement of the husks results in an object that does not burn or even liberate heat in a manner resembling that of any organic substance. These minute silica-crested tubular structures offer an inherent resistance to burning. Often they seal off and prevent the thorough, uniform burning essential to obtaining a desired end-product.”[14]
Anyone who has tried to set a match to loose rice hulls understands how difficult they are to burn. Since air cannot flow freely through a pile of rice hulls to provide the oxygen needed to sustain rapid combustion, they do not easily and cleanly combust. The bulk density of loose rice hulls is similar to that of baled straw, and anyone who has tried to burn a bale of straw understands the problem associated with the availability of oxygen. But the simple availability of oxygen does not explain everything.
As we have noted above, the high percentage of opaline silica within rice hulls is most unusual in comparison to other plant materials, and some scientists say that during the combustion of rice hulls, the silica ash may form a “cocoon” that prevents oxygen from reaching the carbon inside. Other scientists speculate that, since silica and carbon may be partially bonded at the molecular level, silicon carbide is formed during high-temperature combustion, and that the presence of this heat-resisting ceramic impedes the easy combustion of the rice hull.[15] Still other scientists say that at certain temperatures, the molecular bond between the silica and carbon in the hull is actually strengthened, thereby preventing the thorough and uniform burning of the hull.[16] In any case, even if we do manage to ignite a pile a rice hull, we find that it tends to smolder rather than flame.
- Rice husks are flame retarding and, at ordinary temperatures, selfextinguishing. A lighted match, tossed onto a pile of rice husks will generally burn out without producing a self-sustaining flame in the husks.[17]
Conventional cellulose insulation necessitates the addition of large quantities of flame and smolder retardants. The concentration of flame and smolder retardant chemicals (such as boric acid, sodium borate, ammonium sulfate, aluminum sulfate, aluminum trihydrate, mono- or di-ammonium phosphate) in conventional cellulose insulation may reach as high as 40% by weight.[18] These chemicals are expensive to purchase and prepare, and the cellulosic fiber must undergo extensive preparation to receive them.
Surprisingly, rice hulls require no flame or smolder retardants. Nature has freely given to this agricultural waste product all of the combustion properties needed to pass the Critical Radiant Flux Test (ASTM C739/E970-89), the Smoldering Combustion Test (ASTM C739, Section 14), and the Surface Burning Characteristics Test (ASTM E84). Recent testing done by R&D Services indicates an average Critical Radiant Flux (CRF) of 0.29 W/cm2, a smoldering combustion weight loss between 0.03% and 0.07%, a Flame Spread Index (FSI) of 10 and a Smoke Development Index (SDI) of 50. Since US building codes require an FSI of 25 or less, and an SDI of 450 or less, we see that the rice hull easily passed these tests. In its raw and unprocessed state, the rice hull constitutes a Class A or Class I insulation material.
All organic materials will absorb or release moisture until they come into equilibrium with the relative humidity of the surrounding air. The high concentration of opaline silica on the outer surface of the rice hull impedes the atmospheric transfer of moisture into the hull. Also, 2.1% to 6.0% of the rice hull consists of a biopolyester called cutin,[19] which, in combination with a wax produced by the rice plant, forms a highly impermeable barrier. Nature employs several very effective strategies to protect the kernel of rice from the water and high humidity generally associated with the cultivation and growth of this plant.
Consequently, studies done on rice hulls at 25°C indicate that the equilibrium moisture content of rice hulls at 50% relative humidity is at or below 10%, while at 90% relative humidity, the equilibrium moisture content of rice hulls remains at or below 15%.[20] A Moisture Vapor Sorption Test (ASTM C739, Section 12) conducted by R&D Services indicates a gain in weight of only 3.23%. This is well below the moisture content needed to sustain the growth of fungi and mold.
The ASTM Standard Specification for cellulose insulation requires a 28-day test for resistance to fungal growth (see section 10 of ASTM C1497, ASTM C1338, Section 6.6 of ASTM C1149 or Section 11 of ASTM C739). Following these standards, R&D Services inoculated rice hulls with five different fungal species, and the rice hulls passed these tests without the addition of fungicides or any other chemicals.
The high concentration of opaline silica on the outer surface of the rice hull also establishes the effective hardness of the rice hull at roughly the same values as reported for opal (6 on the Mohs scale).[21] However, due to the presence of lignin within the rice hull, this hardness is tempered with flexibility and elasticity. Since the rice hull is hard and yet elastic, it resists settling and compression far better than shredded newspapers. The settling of cellulose insulation in a wall cavity can reduce its installed height by as much as 25%. For this reason it is often necessary to stabilize cellulose insulation by means of polyvinyl acetate or an acrylic adhesive. None of these stabilizing compounds are needed with rice hulls, if firmly vibrated or packed into a wall cavity.
Ordinarily loose rice hulls have an angle of repose of about 35 degrees.[22] But once firmly packed into a wall cavity, their tiny tips, edges and hairs interlock to achieve a negative angle of repose. Due to this peculiar bonding of rice hulls under mild pressure, they stabilize in a very uniform manner, and no further settling is possible. Also, since it is not necessary to add fire-retardants, fungicides or any other chemicals to the rice hull, R&D Services has determined that this benign and stable biomass does not emit offensive odors (ASTM C739). Likewise, R&D Services determined that rice hulls do not corrode aluminum, copper or steel (ASTM C739, Section 9).
With rice hulls, we need not engage in a mining or manufacturing process that generates air pollution, water pollution or erosion.[23] With rice hulls, we need not engage in a manufacturing process that depletes our reserves of fossil fuels (as with polystyrene,[24] polyisocyanurate and polyurethane insulation). With rice hulls, we do not use chlorinebased chemicals such as phosgene, propylene chlorohydrin[25] or any ozone-depleting chlorofluorocarbons.[26] With rice hulls, we do not use urea formaldehyde, and surely none of the phenol formaldehyde used in most fiberglass insulation.[27] With rice hulls, we do not have to worry about the irritability or cacinogenicity of dust and fibers.[28] Moreover, those with acute chemical sensitivity should not have to worry about the offgassing associated with binders in batt insulation, with ink in recycled newspaper or with VOCs released from foam insulation.[29] Since rice hulls require no shredding, hammer-milling, fluffing, fiberizing, binding or stabilizing, they possess, surely in those states where hulls are available, far less embodied energy than even cellulose insulation.[30] Since rice hulls do not burn very easily, they require no flame or smolder retardants, and since they are so tough and durable, nothing prevents them from being used and recycled over and over again.
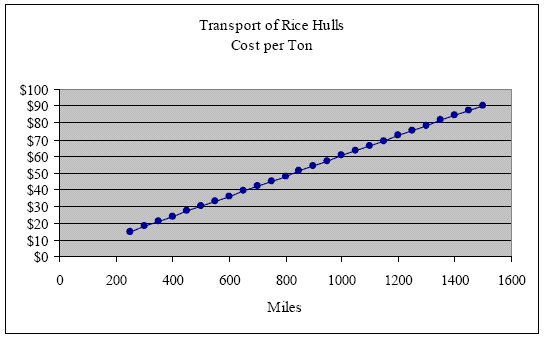
Perhaps the most significant cost associated with the utilization of the rice hull is its transport. At a bulk density of about 9 lbs. per ft3,[31] loose hulls can be transported at roughly the same cost as baled straw. However, to reduce the cost of transport, rice hulls can be compressed to as much as 25 lbs. per ft3 without destroying their elasticity.[32] They readily bounce back to their original density once the force of compression is removed. But to transport rice hulls economically, it would not be necessary to compress rice hulls to a density of 25 lbs/ft3. At a density of only 14.50 lbs/ft3, a standard 53-foot trailer attains optimal transport efficiency at its maximum legal weight of 24 tons. If, at this transport density, we pay an average trucking fee of $1.45 per mile, it would cost approximately $15, $30, $45, $60, $75 and $90 to transport one ton of rice hulls 250, 500, 750, 1000, 1250 and 1500 miles respectively (see graph below).
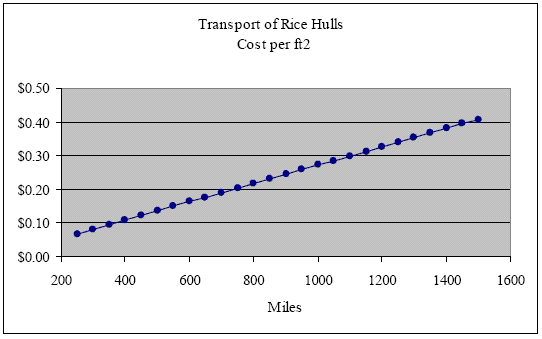
At an installed density of 9 lbs/ft3, one ton of rice hulls will insulate 222 ft2 of a 12-inch wall cavity. Therefore, the cost per ft2 incurred by transport over these same distances is $0.07, $0.14, $0.20, $0.27, $0.34 and $0.41 respectively (see next graph).
Those living less than 200 miles from rice mills should have a hard time justifying the use of any other type of insulation material. When many mills reluctantly sell rice hulls for less than $5.00 dollars per ton, the argument in favor of rice hulls becomes even more compelling. At $5.00 per ton, the cost of rice hulls per ft2 of a 12-inch deep wall is a mere $0.02.
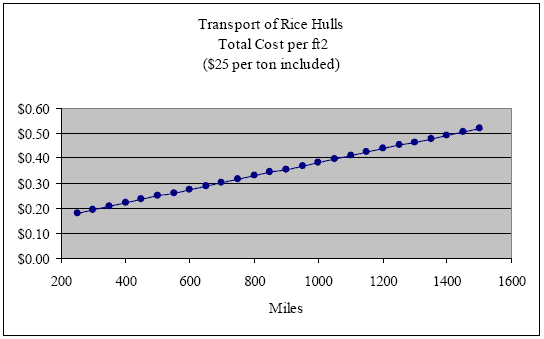
Supposing that we pay not $5.00 but $25 per ton (well above current market value), we find that the purchase price of the rice hulls per ft2 wall insulated is only $0.11. Adding this $0.11 back into the cost of transport over these same distances, we arrive at a total cost per ft2 of the rice hull delivered to the job site of $0.18, $0.25, $0.32, $0.38, $0.45 and $0.52 respectively (see graph below).
With these simple calculations, we see that the transport of rice hulls should not limit or constrain their widespread use as insulation. These calculations allow us to make two comparisons, one with respect to baled straw and the other with respect to dense-pack cellulose insulation. Relative to all other types of insulation on the market today, these two types of insulation possess the highest recycle content and the lowest content of embodied energy.
On average, a two-string straw bale (14x18x36 inches) weighs 45 pounds, sells for $2.50, and transports to the job site for an additional $1.00.[33] Laid flat within a wall, the twostring bale represents 3.5 ft2 of wall surface. This gives a purchase price of $0.71 per ft2 of wall, to which we must add another $0.29 for transport. Accordingly, the total cost of baled straw per ft2 of bale wall is approximately $1.00. This represents more than five times the price of the rice hull transported 250 miles and almost twice the price of the rice hull transported 1,500 miles. Moreover, 12 inches of rice hulls at R-3.0 per inch deliver 37% more insulation than 18 inches of baled straw at R-1.45 per inch,[34] and this, at one fifth to one half the cost, using 33% less wall space.
Cellulose insulation in a dense-pack application is inserted into a wall at a density of approximately 3.5 lbs/ft3. Accordingly, one ton of cellulose insulation will insulate 571 ft2 of our proposed 12-inch deep wall. At an average delivered price of $540 per ton, cellulose insulation costs roughly $0.95 per ft2 of wall insulated. This is slightly cheaper than baled straw, but still roughly five times the price of the rice hull transported 250 miles and twice the price of the rice hull transported 1,500 miles.
If rice hull insulation compares well with straw bale and cellulose insulation, then how much more desirable should it be than those forms of insulation of a low recycle content and a high embodied energy content? The building industry in the United States demands several million tons of insulation on an annual basis. Should rice mills not form an alliance with architects and builders in displacing all forms of insulation not produced in an environmentally efficient and beneficial manner?
- In rebuttal, someone might argue quite correctly that a load-bearing straw bale wall provides a lot more than just insulation. Someone might also argue that we have compared the theoretical insulation value of rice hulls over and against the installed insulation value of straw bales – a classic case of apples and oranges. But insofar as the wall system is correctly designed (no thermal conductivity via structural members), and insofar as the rice hulls are uniformly distributed and packed within the wall (no spaces unoccupied with hulls), the theoretical and installed values should be the same.
continue reading the article on page 2 (due to very large page) right
Appendix


The first rice hull house, completed February, 2004, is the home of Paul and Ly Olivier. Located in the historic steamboat town of Washington, Louisiana, right across from the magnificent Magnolia Ridge Plantation,[35] it is indistinguishable from houses built in the area more than 150 years ago. Many of the building techniques described in this paper have been applied in the construction of this home.
Paul Olivier
Engineering, Separation & Recycling LLC
P.O. Box 250
Washington, Louisiana 70589
Telephone: 1-337-826-5540
E-mail: xpolivier@hotmail.com
Notes
- ↑ Velupillai, L., Mahin, D.B., Warshaw, J.W., and Wailes, E.J. 1996. A Study of the Market for Rice Husk-to-Energy Systems and Equipment, p.24, Louisiana State Agricultural Center. "In nature, silica (SiO2) occurs as seven distinct polymorphs: quartz, cristobalite, tridymite, coesite, stishovite, lechatelerite(silica glass), and opal; the latter two are amorphous." Drees, L., Wilding, L., Smeck, N., and Senkayi, A.1989. Minerals in Soil Environments (2nd Edition), p.913, "Opal is a hydrated silica polymorph(SiO2.nH2O)." Ibid, p.921
- ↑ Rice-Husk Ash Cements: Their Development and Applications, United Nations Industrial Development Organization, Vienna, pp.12-13
- ↑ Juliano, B.1985. Rice: Chemistry and Technology, p. 695
- ↑ "No other plant offal even approaches the amount of silica found in rice husks." Beagle, E.C. 1978. FOA Agricultural Services Bulletin 31, p.8
- ↑ Velupillai (1996), p.1
- ↑ ibid., p.15. See Beagle (1978), pp.6. "Percentages of husk in paddy vary widely, but 20% can be taken as a fair average." Ibid, p.25
- ↑ Velupillai (1996), p.15
- ↑ ibid., p.44
- ↑ ibid., p.37. For a listing of some rice mills in the United States, see http://www.ricecafe.com/newlinks2.htm (deleted site; nov 2010) or ftp://www.usarice.com/publish/member1.htm (requires membership; nov 2010)
- ↑ Velupillai (1996), p.45
- ↑ Velupillai (1996), p.16
- ↑ "Rice hull has a thermal conductivity of about 0.0359 W/(m.°C); the values compare well with the thermal conductivity of excellent insulating materials (Houston, 1972)." Juliano (1985), p.696. The thermal conductivity of rice hull ash is reported to be 0.062 W.m-1.K-1. See UNIDO, p.21. A more recent test done by R&D services of Cookville, Tennessee, indicates a 3.024 R-per-inch.
- ↑ Although charred rice hulls have been sold as an insulation material in loose-fill applications under the trademark name of "Mehabit," it is hard to find evidence that fresh hulls have been used for this purpose. See Beagle (1978), p.132
- ↑ Beagle (1978), p.8. "The high percentage of silica in rice hulls and the peculiar silica-cellulose structure impede uniform and thorough burning of the hulls in a combustion process." Velupillai (1996), p.18. "Of all biomass combustion, the combustion of rice hulls (and straw) is particularly difficult because of the high ash content." Ibid., p.23. "Eldon Beagle set a pile of rice hulls 300'x500'x50' on fire and they burned forsix months." Ibid., p.24. "However, husk cannot be burnt easily or cleanly with excess air, and energyrecovery is very low as the heat produced cannot be utilized in a beneficial manner." Ibid., p.25
- ↑ ibid., p.24
- ↑ From a conversation with Carl D. Simpson of Riceland Foods, Inc
- ↑ Beagle (1978), p.9, quoted from Burrows (109A)
- ↑ "The concentrations of chemicals commonly added in commercial cellulosic insulation normally range from 10 to 40% by weight. Chemicals commonly used are boric acid, sodium borate, ammonium sulfate, aluminum sulfate, aluminum trihydrate, mono- or di-ammonium phosphate." Service Bulletin entitled"Borates for Fire Retardancy in Cellulosic Materials," p.5, prepared by US Borax
- ↑ Juliano (1985), p.695. Regarding cutin[1] (no such page, nov 2010)
- ↑ Juliano (1985), p.707
- ↑ Juliano (1985), p.696
- ↑ Juliano (1985), p.28
- ↑ Much of the comparative language of this paragraph is taken from Environmental Building News – Insulation Materials: Environmental Comparisons[2]
- ↑ "The styrene used in polystyrene insulation is identified by the EPA as a possible carcinogen, mutagen, chronic toxin, and environmental toxin. Further, it is produced from benzene, another chemical with both environmental and health concerns." Ibid, p.5
- ↑ "To manufacture isocyanate, a precursor of polyisocyanurate and polyurethane insulation, two chlorine based chemicals are used: phosgene and propylene chlorohydrin." Ibid., pp.4-5
- ↑ "The most significant pollutants found in insulation materials are chlorine-based chemicals that destroy the earth's protective ozone layer." Ibid., p.5
- ↑ "Most fiberglass insulation is produced using a phenol formaldehyde (PF) binder to hold the fibers together." Ibid., p.5
- ↑ "Growing health concerns about glass fiber" are discussed on p.10 of ibid
- ↑ ibid., pp.10-11
- ↑ Embodied energy is defined as "the energy required to produce and transport materials." Ibid., p.8
- ↑ Juliano (1985), p.696, Velupillai (1996), p.16, Beagle (1978), p.8
- ↑ "Hulls can be readily compressed to about 0.4 g/cm3, and grinding increases bulk density two to four times." Juliano (1985), p.696
- ↑ These figures were submitted by Catherine Wanek, editor of the popular straw bale publication called The Last Straw
- ↑ "Measurements then showed the (straw bale) wall to insulate to R-27.5 (RSI-4.8). On a per-thickness basis, this is R-1.45 per inch (0.099 W/m°C), just about over half of the value most commonly reported."[3] p.2
- ↑ http://www.cajuntravel.com/washington.html