No edit summary |
Sophivorus (talk | contribs) m (Normalize page by renaming "Interwiki links" section to "External links") |
||
| (15 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:Thermodynamics refrigeration.gif|thumb|Simple vapor compression refrigeration diagram]] | |||
'''Refrigeration''' is a process in which work is done to move heat from a low temperature to a high temperature and typically also from one location to another. The work of heat transport is traditionally driven by mechanical work, but can also be driven by heat, magnetism, electricity, laser, or other means. | '''Refrigeration''' is a process in which work is done to move heat from a low temperature to a high temperature and typically also from one location to another. The work of heat transport is traditionally driven by mechanical work, but can also be driven by heat, magnetism, electricity, laser, or other means. | ||
Refrigeration can be used for a variety of purposes: | Refrigeration can be used for a variety of purposes: | ||
* [[refrigerating food]] | * [[refrigerating food]] | ||
* [[cooling]] spaces | * [[cooling]] spaces | ||
An ideal refrigeration system undergoes four different steps during the refrigeration process and comprises four parts. These include the evaporator, the compressor, the condenser, and the expansion valve. Before the compressor and after the evaporator the working fluid is at a low temperature and is isoentropic. After the compressor but before the condenser the working fluid is at high temperature and is still isoentropic. | |||
Going through the condenser the working fluid is entirely isobaric. Before reaching the expansion valve the working fluid is all liquid and is isoenthalpic. After the expansion valve but prior to the evaporator the working valve is some mixture of fluid and gas and is still isoenthaplic. Finally when the working fluid goes through the evaporator it is both isobaric and isothermic. | |||
Different variations of refrigeration systems that exist include an actual vapor-compression refrigeration system, a heat pump, cascade refrigeration systems, multistage compression refrigeration systems, and absorption refrigeration systems, among various others (this is not an exhaustive list). | Different variations of refrigeration systems that exist include an actual vapor-compression refrigeration system, a heat pump, cascade refrigeration systems, multistage compression refrigeration systems, and absorption refrigeration systems, among various others (this is not an exhaustive list). | ||
== Technical == | == Technical == | ||
Terms associated with refrigeration: | Terms associated with refrigeration: | ||
* [[coefficient of performance]] or COP is a measurement of the energy efficiency of a refrigeration (or heat pump) system. | * [[coefficient of performance]] or COP is a measurement of the energy efficiency of a refrigeration (or heat pump) system. | ||
* Q: Heat | |||
* W: Work | |||
* U: Internal Energy, u: Specific Internal Energy | |||
* m<sub>dot</sub>: mass flow rate | |||
* h: enthalpy | |||
COP for refrigeration is calculated as: Cooling effect/Work input, or COP<sub>R</sub>= Q<sub>L</sub>/W<sub>net,in</sub> | COP for refrigeration is calculated as: Cooling effect/Work input, or COP<sub>R</sub>= Q<sub>L</sub>/W<sub>net,in</sub> | ||
where Q<sub>L</sub> is the desired energy into the low temperature environment and W<sub>net,in</sub> is the required work input into the refrigerator itself. | where Q<sub>L</sub> is the desired energy into the low temperature environment and W<sub>net,in</sub> is the required work input into the refrigerator itself. | ||
Carnot efficiency is a theoretical maximum efficiency that can be achieved by running a [[carnot | Carnot efficiency is a theoretical maximum efficiency that can be achieved by running a [[carnot efficiency]]. The theoretical carnot, or n<sub>th</sub>=W<sub>net</sub>/Q<sub>H</sub>, where W<sub>net</sub> equals the work input into the refrigerator and Q<sub>H</sub> equals the energy in the high temperature reservoir. n<sub>th</sub> can also be equated as Q<sub>H</sub>-Q<sub>L</sub>/Q<sub>H</sub>, where Q<sub>H</sub> is the energy in the high temperature reservoir and Q<sub>L</sub> is the energy in the low temperature reservoir. | ||
Flow rate (mass<sub>rate</sub> or m dot) can be equated as: Q<sub>dot</sub>/h<sub>1</sub>-h<sub>4</sub>, where Q<sub>dot</sub> equals the rate of energy used and the different h values signify enthalpy and can be found or interpolated from a thermodynamic table. | Flow rate (mass<sub>rate</sub> or m dot) can be equated as: Q<sub>dot</sub>/h<sub>1</sub>-h<sub>4</sub>, where Q<sub>dot</sub> equals the rate of energy used and the different h values signify enthalpy and can be found or interpolated from a thermodynamic table. | ||
Conversely, Q<sub>dot</sub>=m<sub>dot</sub>(u<sub>out</sub>-u<sub>in</sub>), where the different u values signify internal energy and can be found or interpolated from a thermodynamic table.<br | Conversely, Q<sub>dot</sub>=m<sub>dot</sub>(u<sub>out</sub>-u<sub>in</sub>), where the different u values signify internal energy and can be found or interpolated from a thermodynamic table.<br> | ||
Tons of refrigeration, known as the cooling load or the refrigeration effect, can be equated as: Q<sub>L,dot</sub>= m<sub>dot</sub>(h<sub>1</sub>-h<sub>4</sub>. | Tons of refrigeration, known as the cooling load or the refrigeration effect, can be equated as: Q<sub>L,dot</sub>= m<sub>dot</sub>(h<sub>1</sub>-h<sub>4</sub>). | ||
Refrigeration systems are generally labelled in terms of tons of refrigeration. One ton of refrigeration is equal to 12,000 Btu/hour or 211 kJ/min. | |||
{{Highlight|1=Conversions|2= | |||
: 1 BTU = 778 ft-lbf of work | |||
: 1 Watt = 3.4122 Btu/hour | |||
: 3,412 Btu= 1 kWh | |||
: 1 kWh = 3.6 MJ | |||
: 1 tonne = 1000 kg | |||
: 1 BTU/sec= 1.414 hp}} | |||
== Refrigeration Tables == | |||
* [http://www.cambridge.org/us/download_file/212553/ R134a Tables - PDF Format] | |||
* [[Thermodynamics properties of R134a|R134a Tables - Webpage Format]] | |||
* [http://www.egr.msu.edu:80/classes/me201/somerton/R12Tables.pdf R-12 Tables] | |||
== External links == | |||
* [[wikipedia:Refrigeration]] | * [[wikipedia:Refrigeration]] | ||
* [[Wikipedia:Phase-change incubator|Phase-change incubator]], a low cost way for health workers to incubate microbial samples. | * [[Wikipedia:Phase-change incubator|Phase-change incubator]], a low cost way for health workers to incubate microbial samples. | ||
* [[Wikipedia:Pot-in-pot refrigerator|Pot-in-pot refrigerator]], using clay pots, cloth and water to keep food fresh. | * [[Wikipedia:Pot-in-pot refrigerator|Pot-in-pot refrigerator]], using clay pots, cloth and water to keep food fresh. | ||
{{ | {{Page data}} | ||
[[Category:Engineering]] | [[Category:Engineering]] | ||
[[Category:Food storage]] | [[Category:Food storage]] | ||
[[Category:Heating and cooling]] | [[Category:Heating and cooling]] | ||
[[Category:Refrigeration | [[Category:Refrigeration]] | ||
Latest revision as of 16:34, 1 June 2023
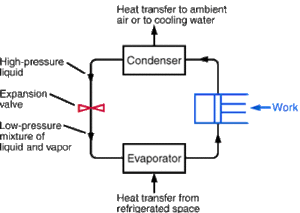
Refrigeration is a process in which work is done to move heat from a low temperature to a high temperature and typically also from one location to another. The work of heat transport is traditionally driven by mechanical work, but can also be driven by heat, magnetism, electricity, laser, or other means.
Refrigeration can be used for a variety of purposes:
- refrigerating food
- cooling spaces
An ideal refrigeration system undergoes four different steps during the refrigeration process and comprises four parts. These include the evaporator, the compressor, the condenser, and the expansion valve. Before the compressor and after the evaporator the working fluid is at a low temperature and is isoentropic. After the compressor but before the condenser the working fluid is at high temperature and is still isoentropic.
Going through the condenser the working fluid is entirely isobaric. Before reaching the expansion valve the working fluid is all liquid and is isoenthalpic. After the expansion valve but prior to the evaporator the working valve is some mixture of fluid and gas and is still isoenthaplic. Finally when the working fluid goes through the evaporator it is both isobaric and isothermic.
Different variations of refrigeration systems that exist include an actual vapor-compression refrigeration system, a heat pump, cascade refrigeration systems, multistage compression refrigeration systems, and absorption refrigeration systems, among various others (this is not an exhaustive list).
Technical[edit | edit source]
Terms associated with refrigeration:
- coefficient of performance or COP is a measurement of the energy efficiency of a refrigeration (or heat pump) system.
- Q: Heat
- W: Work
- U: Internal Energy, u: Specific Internal Energy
- mdot: mass flow rate
- h: enthalpy
COP for refrigeration is calculated as: Cooling effect/Work input, or COPR= QL/Wnet,in where QL is the desired energy into the low temperature environment and Wnet,in is the required work input into the refrigerator itself.
Carnot efficiency is a theoretical maximum efficiency that can be achieved by running a carnot efficiency. The theoretical carnot, or nth=Wnet/QH, where Wnet equals the work input into the refrigerator and QH equals the energy in the high temperature reservoir. nth can also be equated as QH-QL/QH, where QH is the energy in the high temperature reservoir and QL is the energy in the low temperature reservoir.
Flow rate (massrate or m dot) can be equated as: Qdot/h1-h4, where Qdot equals the rate of energy used and the different h values signify enthalpy and can be found or interpolated from a thermodynamic table.
Conversely, Qdot=mdot(uout-uin), where the different u values signify internal energy and can be found or interpolated from a thermodynamic table.
Tons of refrigeration, known as the cooling load or the refrigeration effect, can be equated as: QL,dot= mdot(h1-h4).
Refrigeration systems are generally labelled in terms of tons of refrigeration. One ton of refrigeration is equal to 12,000 Btu/hour or 211 kJ/min.
- 1 BTU = 778 ft-lbf of work
- 1 Watt = 3.4122 Btu/hour
- 3,412 Btu= 1 kWh
- 1 kWh = 3.6 MJ
- 1 tonne = 1000 kg
- 1 BTU/sec= 1.414 hp
Refrigeration Tables[edit | edit source]
External links[edit | edit source]
- wikipedia:Refrigeration
- Phase-change incubator, a low cost way for health workers to incubate microbial samples.
- Pot-in-pot refrigerator, using clay pots, cloth and water to keep food fresh.