J.M.Pearce (talk | contribs) m (→See also) |
J.M.Pearce (talk | contribs) m (→Source) |
||
| Line 3: | Line 3: | ||
[[category:MOST completed projects and publications]] | [[category:MOST completed projects and publications]] | ||
{{Statusboxtop}} | |||
{{status-design}} | |||
{{status-model}} | |||
{{status-prototype}} | |||
{{status-verified|[[MOST]]}} | |||
You can help Appropedia by contributing to the next step in this [[OSAT]]'s [[:Category:Status|status]]. | |||
{{boxbottom}} | |||
==Source== | ==Source== | ||
Revision as of 09:17, 19 May 2020
Template:Statusboxtop Template:Status-design Template:Status-model Template:Status-prototype Template:Status-verified You can help Appropedia by contributing to the next step in this OSAT's status. Template:Boxbottom
Source
- Gallup, N.; Pringle, A.M.; Oberloier, S.; Tanikella, N.G.; Pearce, J.M. Parametric Nasopharyngeal Swab for Sampling COVID-19 and Other Respiratory Viruses: Open Source Design, SLA 3-D Printing and UV Curing System. Preprints 2020, 2020050310 (doi: 10.20944/preprints202005.0310.v1). https://www.preprints.org/manuscript/202005.0310/v1
- All source code on OSF: https://osf.io/z5jgu/
- Swab design NIH:
- UV curing box design on OCI:
- Github repo:
Abstract
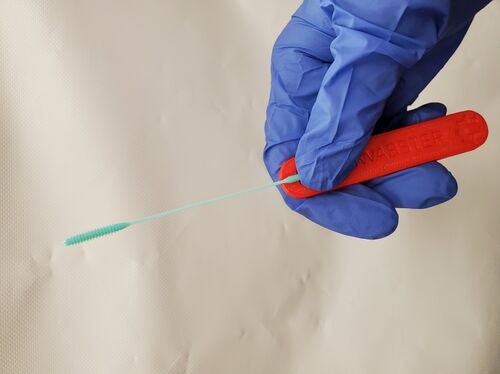
Access to nasopharyngeal swabs for sampling remain a bottleneck in some regions for COVID-19 testing. This study develops a distributed manufacturing solution using only an open source manufacturing tool chain consisting of two types of open source 3-D printing and batch UV curing, and provides a parametric fully free design of a nasopharyngeal swab. The swab was designed using parametric OpenSCAD in two components (a head with engineered break point and various handles), which has several advantages: i) minimizing print time on relatively slow SLA printers, ii) enabling the use of smaller print volume open source SLA printers, iii) reducing the amount of relatively expensive UV resin, and iv) enabling production of handle on more accessible material extrusion 3-D printers. A modular open source UV LED box was designed, fabricated for $45 and tested for batch curing. Swabs can be fabricated for $0.06-$0.12/swab. The results of the mechanical validation tests showed that the swabs could withstand greater forces than would be expected in normal clinical use. The swabs were also able to absorb a significant amounts of synthetic mucus materials and passed abrasion and handling tests. The results show the open source swab are promising candidates for clinical trials.
Keywords
open hardware; COVID-19; medical hardware; nasopharyngeal swab; nasal swab; UV curing; SLA; RepRap; 3-D printing; additive manufacturing
See also
- A review of open source ventilators for COVID-19 and future pandemics
- Maximizing Returns for Public Funding of Medical Research with Open-source Hardware
- Economic Potential for Distributed Manufacturing of Adaptive Aids for Arthritis Patients in the U.S.
- 3-D printing open-source click-MUAC bands for identification of malnutrition
- Emergence of Home Manufacturing in the Developed World: Return on Investment for Open-Source 3-D Printers
- Quantifying the Value of Open Source Hardware Development
- Distributed Manufacturing of Open-Source Medical Hardware for Pandemics





