The purpose of this page is to describe the solar hot water system used by the Campus Center for Appropriate Technology (CCAT) on the campus of Humboldt State University (HSU).

CCAT Overview
Developed as a live in laboratory for sustainability, the student run, student funded, and student staffed organization is now located in a residential style house on the south side of campus(Figure 1). Since becoming an associated students program in 1980, CCAT has been organized around the goal of providing appropriate technology information to the public.
One example of appropriate technology featured at CCAT is the solar hot water system, which provides hot water for the entire house using the thermal energy from the sun and is considered to be the most functionally efficient system still operating at CCAT as of October 2011.
Solar Hot Water System
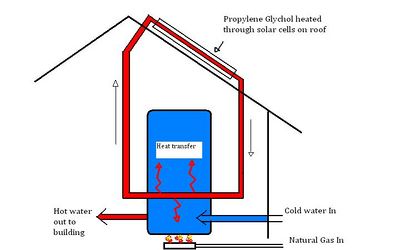
In July of 2009, two solar-thermal cells were installed on the roof of the CCAT house and connected to the water heater for the building. Figure 2 shown below has been simplified for conceptual purposes. The essential components of the system are the Phoenix high-efficiency water heater, the heating agent propylene glycol and the solar cells, which heat the propylene glycol through solar radiation.
- Biodegradable propylene glycol[1] is circulated in a closed loop system and heated via solar radiation within the two solar thermal cells on the roof of the building.
- The heated propylene glycol is then circulated through a heat transfer coil specially designed to maximize surface area (therefore increasing the rate of heat transfer) which "Preheats" or warms the cold water in the tank via thermal conduction.
- This water is then brought up to the final desired thermostat temperature by a conventional natural gas burner and distributed to the building through conventional domestic plumbing.
- Also included in the system ,though not pictured here, is a closed loop hydronic heating path run through the cement floors dedicated to heating the building.
Components
The Phoenix High Efficiency Water Heater

Shown in Figure 3, the Phoenixis a high-efficiency water/space heater powered by either liquid propane or in the case CCAT, natural gas. The system is designed and manufactured by Heat Transfer. By itself the Phoenix offers several advantages over a standard water heater. [2]
- When run purely on gas, devoid of solar augmentation, the Phoenix features a combustion efficiency of 96%.
- Emissions from combustion contain much lower concentrations of NOx.
- Modulating or "load matching" burner works harder when demand is up and less when demand is down. A conventional burner will heat the water at full capacity regardless of demand.
- Heat exchange from the burner and additional captured heat from its processes is so efficient that exhaust can be vented using PVC conduit.
- The unit is pre-plumbed for connection to solar-thermal systems and/or hydronic heating elements.
Solar-Thermal Cells
The two cells, dedicated to the solar hot water system at CCAT, are manufactured by Schueco; a German company that designs and distributes both components and complete systems designed to capture solar energy. The cells feature highly efficient thermal insulation, absorbent coatings and copper piping which circulates a biodegradable heating agent. The cells utilize the sun's energy to heat, through solar radiation, the heating agent propylene glycol. When connected and properly plumbed the system is a closed loop. The two cells face south in order to achieve maximum sunlight exposure, as seen near the top of the CCAT roof in Figure 1 (the upper-most two panels located on the roof).
Heating Agent-Propylene Glycol
The heating agent in this case is a biodegradable product similar to the antifreeze used in automobiles. CCAT staff have monitored propylene glycol temperatures up to 135 degrees Fahrenheit through in-line gauges. When digital monitors sense the temperature of the propylene glycol drop below the temperature of the water in the water heater tank, at night for example, the circulation is automatically shut off to prevent cooling of the water.
Funding
CCAT received funding for this project through a grant allocated by the Humboldt Energy Independence Fund (HEIF)[3]. The grant proposalsubmitted by the student directors has a total proposed cost not to exceed $14,951. This figure does not account for future costs and/or upkeep but does take into account labor costs for installation. The proposal cites May of 2010 as the date for total project completion[4].
Remaining Issues
As a result of the short time in operation and only partial completion of the solar hotwater project, the following issues were not addressed in the above sections.
- Hydronic heating system
- Propylene Glycol disposal
No Longer Remaining Issues
- Monitoring equipment
- Sufficient data for determining efficiency of the system
using data from 2010 it was determined that the solar water system resulted in the use of only 347 therms over the course of the whole year. The average household in California uses around 800 Therms (not considering colder climates). Assuming this average consumption, the solar water system in use at CCAT has saved approximately $212.50 for the year of 2010.
References
- ↑ Propylene Glycol. (n.d.) In Wikipedia. Retrieved on October 22, 2009, from http://en.wikipedia.org/wiki/Propylene_glycol
- ↑ Heat Transfer. (2007, February 7). Product Release. Retrieved October, 14, 2009, from http://www.htproducts.com/literature/pr-phoenix.pdf
- ↑ HSU Energy Independence Fund. Retrieved on October26, 2009, from http://www.humboldt.edu/~heif/
- ↑ Hughes, L. and Steuben, J. (2008, November 11). Proposal Title: Solar Thermal Project Fall 2008. Retrieved on October12, 2009 from http://www.humboldt.edu/%7Eheif/proposals/CCAT_solar_thermal_fall_08.pdf
6. Davis, M., & Masten, S. (2009). Principles of Environmental Engineering and Science. (Second ed.).(pg. 159). New York, NY: McGraw-Hill Companies. Print.