Arcata's oxidation ponds are facultative ponds, which are used primarily by small communities for the removal of organic wastes from waste water. Oxidation ponds consist of about 25% of America's municipal waste water treatment facilities, since maintenance and removal is inexpensive. The process involves first introducing wastewater, which contains "suspended solids" (colloidW) The insoluble solids, referred to as settable solids (dead algae and other compounds) in Figure 2 sink to the bottom of the pond to promote an anaerobic layer of bacteria.[1] Above this layer and at the water surface, aerobicW bacteria exist due to the sunlight and presence of oxygen from photosynthesis. The combination of the two bacterial layers create a facultative pond due to the varying penetration of light throughout the pond. The facultative bacteria inhabit the center of the pond, and are able to acquire energy both aerobically and anaerobically from compounds like sulfates and phosphates illustrated in Figure 2 below.
The oxygen required to sustain life is ultimately coming from the sun, because this light ensures photosynthesis producing oxygen, but what about at night? The source of oxygen is acquired by diffusion across the water surface. Arcata's oxidation ponds have an aerator that increase the oxygen so that the ponds do not become anaerobic. The ponds would function improperly as a wastewater treatment technique if they were to become anaerobic because of the slow consumption rates and release of harmful gases.
Characteristics of Oxidation Ponds[edit | edit source]
Aerobic ponds are only a meter in depth and filled with aerobic bacteria (Bacillus, cyanobacteria, Nocardia). The BOD (Biochemical Oxygen DemandW),which is the water quality of the pond, has to be no more than 500 mg/L. The quantities of waste should be dilute to keep the pond at its highest efficiency.[1] Fully aerobic ponds with BOD values higher than 500mg/L are hard to sustain because of the inability of sufficiently aerating the pond, and the production of biological sludge that eventually turns anaerobic depicted in Figure 2.
Anaerobic ponds are lagoons containing mostly anaerobic bacteria (Acteroides Fragilis, and Fusobacterium). They are intolerant to oxygen and require that oxygen levels are very low around 0.2 mg/L.[2] They are greater in depth than aerobic ponds to allow for low light penetration, so that photosynthesis can't be initiated throughout. When the ponds are introduced to high concentrations of organic matter the transparency of the water decreases contributing to low dissolved oxygen levels from the lack of photosynthesis. Depths of 2-5 meters and high waste content give a suitable environment for these bacteria. A BOD concentration of no greater than 1000 mg/L is desirable to sustain anaerobic conditions.[1]
The ability of anaerobic microorganisms to process waste is dependent on the climate. Differences in weather conditions, such as, temperature and wind affect the metabolic rates by distributing solids among the ponds. Wind provides too much aeration within the pond. Excessive aeration can impose danger to the anaerobic bacteria, because of the possibility of oxygen being more readily present, which would kill them.[1]
Here are some fundamental factors considered to make oxidation ponds function properly as a treatment process:
- Lagoon depth
- Bacterial species
- Composition of wastes
- Amount of wastes[1]
- Temperature at 20 C
- pH within 6-8
Removal Processes[edit | edit source]
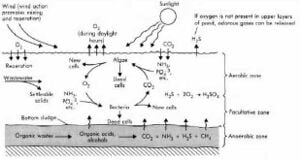
AEROBIC ZONE END PRODUCTS
Amino acids + Ammonia + O2 => Nitrites => Nitrates Organic compounds + O2 => CO2 + H2O CO2 + LIGHT + H2O => C6H12O6 + O2 + Algae (photosynthesis)
ANAEROBIC ZONE END PRODUCTS
Organic compounds = Organic acids + Alcohols (Acid Fermentation) Organic acids + Alcohols = CH4 + CO2 (Methane Ferm.)[1]
Aerobic bacteria have the ability to consume much of the soluble suspended solids. They are able to remove these compounds at a rate 90 percent faster than anaerobic bacteria.[3] This means that treatment requires 90 percent less aerobic bacteria than anaerobic bacteria, or if you had an equivalent amount of anaerobic bacteria the aerobic bacteria would digest the organic matter 90 percent faster. Both aerobic and anaerobic bacteria consume different substrates than each other, thus both bacteria types are present to facilitate a facultative pond for greater efficiency.
Many of the suspended solids are in the form of proteins and carbohydratesW. The text beside of Figure 2 describes the compounds being consumed and discharged through oxidationW processes conducted by the microorganisms. Ammonia and amino acids are nitrogen based compounds, so that the aerobic bacteria most easily converts these complex compounds into nitratesW.[1] The nitrates produced and released into the water are harmless non-toxic compounds and promote algal growth. Organic compounds that contain phosphorus and sulfur can be converted into simpler compounds referred to as phosphates and sulfates, presented in Figure 2.
The equations to the right of Figure 2 expresses the main processes undergone by both anaerobic bacteria and aerobic bacteria. Anaerobic bacteria goes through a two staged process to consume organic matter. The first stage is referred to as acid fermentation, which is the breakdown of complex organic molecules into long chains of alcohols and organic acids (lactic acid, and many other carboxylW containing compounds). The next stage is called methane fermentation, which is when the alcohols are converted to gaseous methane and carbon dioxide (consumed in photosynthesis). Anaerobic processes also produce many other compounds, such as hydrogen sulfide, ammonia, mercaptansW, and carbon dioxide.[1] Only 20%-30% of the ammonia produced is released into the atmosphere as gas, and about 30% of all the effluent ultimately gets converted into methane gas.
Arcata[edit | edit source]
Arcata's oxidation ponds were built in 1957 and have not been drained in their lifetime. It was separated into two ponds by a dike in 1986 one is 9.9 hectares and the other is 6.9 hectares.[2] Arcata's two oxidation ponds that while having the same depth (2m), vary in area. The decomposition of the variety of solids including soluble and insoluble, were evenly dispersed between both bodies of water resulting in a uniform mass to volume ratio. Pond one effectively removes 77 percent of organic wastes and pond two consumes about 79 percent. Table 1 quantifies the high removal efficiency of organic solids with a BOD of 2-300 mg/L. Average efficiencies reach along the lines of about 60% to 80%.[4]
| Total Initial Mass(tons) | Mean % Reduction | Total Solids Remaining | |
|---|---|---|---|
| Oxidation Pond One | 229 tons | 77% | 51.1 tons |
| Oxidation Pond Two | 113 tons | 79% | 25.2 tons |
The efficiency was due to trenches put in place along the walls of the dike when the oxidation ponds were constructed in 1957. The trenches are unique to Arcata's oxidation ponds, which add an extra meter in depth to facilitate the growth of large amounts of anaerobic bacteria. The trench sites allow for a large accumulation and compaction of organic solids. The trench also acts as an anaerobic digestor within the facultative oxidation pond itself.[2]
References[edit | edit source]
- Hamzeh Ramadan and Victor M. Ponce (2008). Design and Performance of Waste Stabilization Ponds. Version 080714. Retrieved Dec 06 2008. http://web.archive.org/web/20190720151154/http://stabilizationponds.sdsu.edu:80/
- Anaerobic Organism (2008). Accessed Online Nov 14 2008. http://en.wikipedia.org/wiki/Anaerobic_organism
- Norweco (2006). Norwalk Wastewater Equipment Company, Inc. Identification of Wastewater Bacteria. Retrieved Nov 14, 2008. http://www.norweco.com/html/lab/Identify.htm
- IRC (2003). Waste stabilization ponds for wastewater treatment. IRC. Retrieved Dec 06 2008. http://web.archive.org/web/20121209062746/http://www.irc.nl:80/page/8237