| Line 24: | Line 24: | ||
====Direction==== | ====Direction==== | ||
Reusing lead | |||
"[https://www.youtube.com/watch?v=bBjrod-oopM Recycling lead acid batteries into perovskite solar cells.]" | "[https://www.youtube.com/watch?v=bBjrod-oopM Recycling lead acid batteries into perovskite solar cells.]" | ||
[[Open-source syringe pump]] | Used for syringe design | ||
[[Open-source syringe pump]] | |||
Used for Raspberry Pi set up on prusa for franklin | |||
[[How to install FLIR Lepton Thermal Camera and applications on Raspberry Pi]] | |||
====Basic Knowledge==== | ====Basic Knowledge==== | ||
Revision as of 20:21, 1 December 2015
Purpose
This literature review is meant to provide an understanding of the usefulness and properties of perovskites as a material for 3D printing solar cells.
Current Design
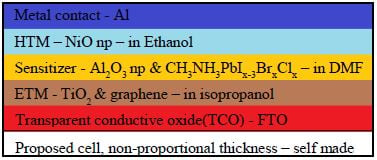
- Metal contact - Aluminum Metal
- Hole Transporting Material - NiO np
- Sensitier - CH3NH3PbIx-3BrxClx
- Mesoscopic Layer - Al2O3 np
Al2O3 gamma np - Reasoning for gamma over alpha: highly porous, potentially catalytic features - need paper citation
- Electron Transporting Material - TiO2 & graphene flakes
- Transparent Conducting Oxide - Florine doped Tin-Oxide
Direction
Reusing lead "Recycling lead acid batteries into perovskite solar cells."
Used for syringe design Open-source syringe pump
Used for Raspberry Pi set up on prusa for franklin How to install FLIR Lepton Thermal Camera and applications on Raspberry Pi
Basic Knowledge
Perovskite
Solar Energy
3D printing
Papers Found
Review Articles Group 1
Perovskite solar cells: an emerging photovoltaic technology
Source and full text: Nam-Gyu Park, “Perovskite solar cells: an emerging photovoltaic technology”,Materials Today 18, pp. 65-72 (2015).
- Article includes history of Perovskite technology
- The ABX3 structure is discussed. (X=O,C,N,halogen) X anion is most effective as a halogen. Emphasis on the B cation octahedral and A cation cubo-octahedral structure.
- Carrier diffusion lengths found to be greater than one micron for both electrons and holes.
- A architecture of FTO/bl-TiO2/MAPbI3/Au resulted in a PCE of 8%. Devoid of a mesopourous TiO2 layer and HTM. (MA = CH3NH3)
- A mixed halide perovskite (MAPbI3-xBrx) (x=0-3) appeared more stable in moisture. Br is suspected to stabilize the Ch3NH3+ cation in the lattice.
- A mixed halide perovskite (I,Br,Cl) would also allow the tuning of the band gap for greater light absorption.
Current progress and future perspectives for organic/inorganic perovskite solar cells
Source and full text: Pablo P. Boix, Kazuteru Nonomura, Nripan Mathews, Subodh G. Mhaisalkar “Current progress and future perspectives for organic/inorganic perovskite solar cell”,Materials Today 17, pp. 16-23 (2014).
- In general: iodides cause a smaller bandgap and longer wavelength light emission, while bromides cause a higher bandgap and shorter wavelength light emission.
- MAPb3 has a bandgap of 1.55eV, optimum is about 1.4eV
- The compact TiO2 layer is needed for collecting the generated electrons and blocking holes.
- Because the Al2O3 conduction band is higher than the absorber's LUMO, no electron injection from the perovskite takes place, indicating that the electron transport occurs within the perovskite itself.
- MAPbI3 has a high extinction coefficient, which ensures a good absorption of light at low mesoporous film thickness
- the crystallizing nature of perovskite upon deposition is important for both conductivity and charge generation since the crystallinity determines the distribution of energetic states.
- Used archetecture: Glass/FTO/TiO2-bl/Al2O3 scafold/MAPbI3/Spiro-OMeTAD/metal contact resulted in a PCE of 10.9%
- TiO2/CH3NH3PbI3/Au contact resulted in a PCE of 5.5%
The light and shade of perovskite solar cells
Source and Full Text: Michael Grätzel The light and shade of perovskite solar cells", Nature Materials 13, pp. 838–842, (2014).
- Abstract: The rise of metal halide perovskites as light harvesters has stunned the photovoltaic community. As the efficiency race continues, questions on the control of the performance of perovskite solar cells and on its characterization are being addressed.
Organolead halide perovskite: A rising player in high-efficiency solar cells
Source and Text through ILLiad Interlibrary Loan: Zhou Yang, Wen-Hua Zhang,"Organolead halide perovskite: A rising player in high-efficiency solar cells", Chinese Journal of Catalysis 35, pp. 983-988, (2014).
- Abstract: This perspective presents a brief description of organolead halide perovskite-based solar cells, including the structures and fundamental properties of perovskite, classifications of solar cells, and outlook of their potentials as subcells of tandem photovoltaic devices and large scale applicability.
Trend of Perovskite Solar Cells: Dig Deeper to Build Higher
Source and Full Text: Kai Zhu, Tsutomu Miyasaka, Jin Young Kim, and Iván Mora-Seró,"Organolead halide perovskite: A rising player in high-efficiency solar cells", J. Phys. Chem. Lett. 6, pp. 2315–2317, (2015).
- single-junction PSC reached Certified 20.1% PCE.
- controlling nucleation and grain growth may provide a more compact and uniform perovskite layer.
- Cl addition adds blue-shifted luminescence and band gap control. (multiple halides term it a wide band-gap cell)
- A hysteresis-free J-V curve can be performed at either very slow or very fast scan rates. Hysteresis during J-V measurements can lead to false PCE values. Additionally, stabilized output at max power should be checked.
- large stability issue for MAPbI3 as in the precence of water it will decompose into PbI2 and CH3NH3I. In the dark it forms a hydrate: MA4PbI6·2H2O. However, this stability can be mitigated by using a mixed halide design (doping the perovskite with Br and Cl).
A brief history of perovskite materials for photovoltaic applications
Source and Full Text: 1. P. Gao, M. Grätzel, and M. K. Nazeeruddin, "Organohalide lead perovskites for photovoltaic applications", Energy & Environmental Science. 7, pp. 2448, (2014)..
Focused Papers-Solar Cell Group 1
Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells
Source and Text through ILLiad Interlibrary Loan: Nam Joong Jeon,Jun Hong Noh,Young Chan Kim,Woon Seok Yang,Seungchan Ryu & Sang Il Seok,"Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells", Nature Materials 13, pp. 897–903, (2014).
- Abstract: Organolead trihalide perovskite materials have been successfully used as light absorbers in efficient photovoltaic cells. Two different cell structures, based on mesoscopic metal oxides and planar heterojunctions have already demonstrated very impressive advances in performance. Here, we report a bilayer architecture comprising the key features of mesoscopic and planar structures obtained by a fully solution-based process. We used CH3NH3 Pb(I1 − xBrx)3 (x = 0.1–0.15) as the absorbing layer and poly(triarylamine) as a hole-transporting material. The use of a mixed solvent of γ-butyrolactone and dimethylsulphoxide (DMSO) followed by toluene drop-casting leads to extremely uniform and dense perovskite layers via a CH3NH3I–PbI2–DMSO intermediate phase, and enables the fabrication of remarkably improved solar cells with a certified power-conversion efficiency of 16.2% and no hysteresis. These results provide important progress towards the understanding of the role of solution-processing in the realization of low-cost and highly efficient perovskite solar cells.
High efficiency CH3NH3PbI(3−x)Clx perovskite solar cells with poly(3-hexylthiophene) hole transport layer
Source and Full Text: Francesco Di Giacomo, Stefano Razza, Fabio Matteocci, Alessandra D'Epifanio, Silvia Licoccia, Thomas M. Brown, Aldo Di Carlo,"High efficiency CH3NH3PbI(3−x)Clx perovskite solar cells with poly(3-hexylthiophene) hole transport layer", Journal of Power Sources 251, pp. 152–156, (2014).
- Not useful for this project. Organic HTMs are have very poor stability. They tend to absorb moisture and allow the perovskite itself to come into contact with moisture which leads to degradation of the cell. PCE significantly diminishes.
Influence of compact TiO2 layer on the photovoltaic characteristics of the organometal halide perovskite-based solar cells
Source and Text through ILLiad Interlibrary Loan: Xiaomeng Wang, Yanling Fang, Lei He, Qi Wang, Tao Wu,"Influence of compact TiO2 layer on the photovoltaic characteristics of the organometal halide perovskite-based solar cells", Materials Science in Semiconductor Processing 27, pp. 569-576, (2014).
- Abstract: A series of perovskite-based solar cells were fabricated wherein a compact layer (CL) of TiO2 of varying thickness (0–390 nm) was introduced by spray pyrolysis deposition between fluorine-doped tin oxide (FTO) electrode and TiO2 nanoparticle layer in perovskite-based solar cells. Investigations of the CL thickness-dependent current density–voltage (J–V) characteristics, dark current, and open circuit voltage (Voc) decays showed a similar trend for thickness dependence. A CL thickness of 90 nm afforded the perovskite-based solar cell with the maximum power conversion efficiency (η, 3.17%). Furthermore, two additional devices, perovskite-based solar cell omitting hole transporting materials layer and cell without the TiO2 nanoparticles, were designed and fabricated to study the influence of the CL thickness on different electron transport paths in perovskite-based solar cells. Solar cells devoid of TiO2 nanoparticles, but with perovskite and organic hole-transport materials (HTMs), exhibited sustained improvement in photovoltaic performances with increase in the thickness of CL, which is in contrast to the behavior of classical perovskite-based solar cell and common solid state solar cell which showed optimal photovoltaic performances when the thickness of CL is 90 nm. These observations suggested that TiO2 nanoparticles play a significant role in electron transport in perovskite-based solar cells.
Gas-assisted preparation of lead iodide perovskite films consisting of a monolayer of single crystalline grains for high efficiency planar solar cells
Source and Text through ILLiad Interlibrary Loan: Fuzhi Huang, Yasmina Dkhissi, Wenchao Huang, Manda Xiao, Iacopo Benesperi, Sergey Rubanov, Ye Zhu, Xiongfeng Lin, Liangcong Jiang, Yecheng Zhou, Angus Gray-Weale, Joanne Etheridge, Christopher R. McNeill, Rachel A. Caruso, Udo Bacha, Leone Spiccia, Yi-Bing Cheng,"Gas-assisted preparation of lead iodide perovskite films consisting of a monolayer of single crystalline grains for high efficiency planar solar cells", Nano Energy 10, pp. 10-18, (2014).
- Not useful for this project.
Charge Transport and Recombination in Perovskite (CH3NH3)PbI3 Sensitized TiO2 Solar Cells
Source and Full Text: Yixin Zhao and Kai Zhu,"Charge Transport and Recombination in Perovskite (CH3NH3)PbI3 Sensitized TiO2 Solar Cells", J. Phys. Chem. Lett. 4, pp. 2880–2884, (2013).
- Abstract: We report on the effect of TiO2 film thickness on the charge transport, recombination, and device characteristics of perovskite (CH3NH3)PbI3 sensitized solar cells using iodide-based electrolytes. (CH3NH3)PbI3 is relatively stable in a nonpolar solvent (e.g., ethyl acetate) with a low iodide concentration (e.g., 80 mM). Frequency-resolved modulated photocurrent/photovoltage spectroscopies show that increasing TiO2 film thickness from 1.8 to 8.3 μm has little effect on transport but increases recombination by more than 10-fold, reducing the electron diffusion length from 16.9 to 5.5 μm, which can be explained by the higher degree of iodide depletion within the TiO2 pores for thicker films. The changes of the charge-collection and light-absorption properties of (CH3NH3)PbI3 sensitized cells with varying TiO2 film thickness strongly affect the photocurrent density, photovoltage, fill factor, and solar conversion efficiency. Developing alternative, compatible redox electrolytes is important for (CH3NH3)PbI3 or similar perovskites to be used for potential photoelectrochemical applications.
Optical bleaching of perovskite (CH3NH3)PbI3 through room-temperature phase transformation induced by ammonia
Source and Full Text: Yixin Zhao and Kai Zhu,"Optical bleaching of perovskite (CH3NH3)PbI3 through room-temperature phase transformation induced by ammonia", Chemical Communications 13, pp. 1605-1607, (2014).
- Not useful for this project
Solid-State Mesostructured Perovskite CH3NH3PbI3 Solar Cells: Charge Transport, Recombination, and Diffusion Length
Source and Full Text: Yixin Zhao, Alexandre M. Nardes, and Kai Zhu,"Solid-State Mesostructured Perovskite CH3NH3PbI3 Solar Cells: Charge Transport, Recombination, and Diffusion Length", J. Phys. Chem. Lett. 5, pp. 490–494, (2014).
- Abstract: We report on the effect of TiO2 film thickness on charge transport and recombination in solid-state mesostructured perovskite CH3NH3PbI3 (via one-step coating) solar cells using spiro-MeOTAD as the hole conductor. Intensity-modulated
photocurrent/photovoltage spectroscopies show that the transport and recombination properties of solid-state mesostructured perovskite solar cells are similar to those of solidstate dye-sensitized solar cells. Charge transport in perovskite cells is dominated by electron conduction within the mesoporous TiO2 network rather than from the perovskite layer. Although no significant film-thickness dependence is found for transport and recombination, the efficiency of perovskite cells increases with TiO2 film thickness from 240 nm to about 650−850 nm owing primarily to the enhanced light harvesting. Further increasing film thickness reduces cell efficiency associated with decreased fill factor or photocurrent density. The electron diffusion length in mesostructured perovskite cells is longer than 1 μm for over four orders of magnitude of light intensity.
Low-Temperature and Solution-Processed Amorphous WOX as Electron-Selective Layer for Perovskite Solar Cells
Source and Full Text: Kai Wang, Yantao Shi, Qingshun Dong, Yu Li, Shufeng Wang, Xufeng Yu, Mengyao Wu, and Tingli Ma,"Low-Temperature and Solution-Processed Amorphous WOX as Electron-Selective Layer for Perovskite Solar Cells", J. Phys. Chem. Lett. 6, pp. 755–759, (2015).
- Study demonstrates WOx is an alternative to TiO2 for the ETM material.
Crystal Morphologies of Organolead Trihalide in Mesoscopic/Planar Perovskite Solar Cells
Source and Full Text: Yuanyuan Zhou, Alexander L. Vasiliev, Wenwen Wu, Mengjin Yang, Shuping Pang, Kai Zhu, and Nitin P. Padture,"Crystal Morphologies of Organolead Trihalide in Mesoscopic/Planar Perovskite Solar Cells", J. Phys. Chem. Lett. 6, pp. 2292–2297, (2015).
- Abstract: The crystal morphology of organolead trihalide perovskite (OTP) light absorbers can have profound influence on the perovskite solar cells (PSCs) performance. Here we have used a combination of conventional transmission electron microscopy (TEM) and high-resolution TEM (HRTEM), in cross-section and plan-view, to characterize the morphologies of a solution-processed OTP (CH3NH3PbI3 or MAPbI3) within mesoporous TiO2 scaffolds and within capping and planar layers. Studies of TEM specimens prepared with and without the use of focused ion beam (FIB) show that FIBing is a viable method for preparing TEM specimens. HRTEM studies, in conjunction with quantitative X-ray diffraction, show that MAPbI3 perovskite within mesoporous TiO2 scaffold has equiaxed grains of size 10–20 nm and relatively low crystallinity. In contrast, the grain size of MAPbI3 perovskite in the capping and the planar layers can be larger than 100 nm in our PSCs, and the grains can be elongated and textured, with relatively high crystallinity. The observed differences in the performance of planar and mesoscopic-planar hybrid PSCs can be attributed in part to the striking differences in their perovskite-grain morphologies.
Hole-Conductor-Free, Metal-Electrode-Free TiO2/CH3NH3PbI3 Heterojunction Solar Cells Based on a Low-Temperature Carbon Electrode
Source and Full Text: Huawei Zhou, Yantao Shi, Qingshun Dong, Hong Zhang, Yujin Xing, Kai Wang, Yi Du, and Tingli Ma,"Hole-Conductor-Free, Metal-Electrode-Free TiO2/CH3NH3PbI3 Heterojunction Solar Cells Based on a Low-Temperature Carbon Electrode", J. Phys. Chem. Lett. 5, pp. 3241–3246, (2014).
- Abstract: Low cost, high efficiency, and stability are straightforward research challenges in the development of organic–inorganic perovskite solar cells. Organolead halide is unstable at high temperatures or in some solvents. The direct preparation of a carbon layer on top becomes difficult. In this study, we successfully prepared full solution-processed low-cost TiO2/CH3NH3PbI3 heterojunction (HJ) solar cells based on a low-temperature carbon electrode. Power conversion efficiency of mesoporous (M-)TiO2/CH3NH3PbI3/C HJ solar cells based on a low-temperature-processed carbon electrode achieved 9%. The devices of M-TiO2/CH3NH3PbI3/C HJ solar cells without encapsulation exhibited advantageous stability (over 2000 h) in air in the dark. The ability to process low-cost carbon electrodes at low temperature on top of the CH3NH3PbI3 layer without destroying its structure reduces the cost and simplifies the fabrication process of perovskite HJ solar cells. This ability also provides higher flexibility to choose and optimize the device, as well as investigate the underlying active layers.
Review Articles Group 2
Advancements in all-solid-state hybrid solar cells based on organometal halide perovskites
Source and Full Text: Shaowei Shi, Yongfang Li, Xiaoyu Li, and Haiqiao Wang, "Advancements in all-solid-state hybrid solar cells based on organometal halide perovskites", Material horizons 2 pp. 378-405, (2015).
- Very useful review article. Includes various up to date deposition methods for PSCs.
- Perovskites can act as a light absorber as well as a bipolar transport of both holes and electrons.
- In depth description of benefits of potential device architectures for PSCs.
- Mesoporous metal oxide n-type layers like TiO2. FTO/bl-TiO2/mp-TiO2(rutile)/MAPbX3/spiro-MeOTAD/Au (X=I,Br,Cl)
- Meso-superstructured designs using Al2O3. FTO/bl-TiO2/mp-Al2O3/MAPbX3/spiro-MeOTAD/Ag (X=I,Br,Cl)
- Various HTMs are discussed, Spiro-MeOTAD so far contributes to the highest PCE, however, P3HT, and PTAA are viable polymer based HTMs.
- Main limiting factor for practical application is the sensitivity to moisture and elevated temperature.
Solution Chemistry Engineering toward High-Efficiency Perovskite Solar Cells
Source and Full Text: Yixin Zhao and Kai Zhu,"Solution Chemistry Engineering toward High-Efficiency Perovskite Solar Cells", J. Phys. Chem. Lett. 5, pp. 4175–4186, (2014).
- Abstract: Organic and inorganic hybrid perovskites (e.g., CH3NH3PbI3) have emerged as a revolutionary class of light-absorbing semiconductors that has demonstrated a rapid increase in efficiency within a few years of active research. Controlling perovskite morphology and composition has been found critical to developing high-performance perovskite solar cells. The recent development of solution chemistry engineering has led to fabrication of greater than 15–17%-efficiency solar cells by multiple groups, with the highest certified 17.9% efficiency that has significantly surpassed the best-reported perovskite solar cell by vapor-phase growth. In this Perspective, we review recent progress on solution chemistry engineering processes and various control parameters that are critical to the success of solution growth of high-quality perovskite films. We discuss the importance of understanding the impact of solution-processing parameters and perovskite film architectures on the fundamental charge carrier dynamics in perovskite solar cells. The cost and stability issues of perovskite solar cells will also be discussed.
Review of recent progress in chemical stability of perovskite solar cells
summary pending
Focused Papers-Solar Cell Group 2
Summaries to be added
Efficient organic–inorganic hybrid perovskite solar cells processed in air
Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells
Sub-150C processed meso-superstructured perovskite solar cells with enhanced efficiency
Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates
Carbon Nanotube/Polymer Composites as a Highly Stable Hole Collection Layer in Perovskite Solar Cells
Efficient planar heterojunction perovskite solar cells by vapour deposition
Environmentally responsible fabrication of efficient perovskite solar cells from recycled car batteries
Low-Temperature Processed Electron Collection Layers of Graphene/TiO2 Nanocomposites in Thin Film Perovskite Solar Cells
p-type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells
New Physical Deposition Approach for Low Cost Inorganic Hole Transport Layer in Normal Architecture of Durable Perovskite Solar Cells
Metal/Metal-Oxide Interfaces: How Metal Contacts Affect the Work Function and Band Structure of MoO3
Small Photocarrier Effective Masses Featuring Ambipolar Transport in Methylammonium Lead Iodide Perovskite: A Density Functional Analysis
Comparison of transparent conductive indium tin oxide, titanium-doped indium oxide, and fluorine-doped tin oxide films for dye-sensitized solar cell application
Focused Papers-3D Printing
Open-Source Syringe Pump Library
Combining 3D printing and liquid handling to produce user-friendly reactionware for chemical synthesis and purification