| health-topic = Maternal mortality | health-classification = Diagnosis }}
Problem being addressed[edit | edit source]
Accurate measurement of vital signs is important to properly monitor patients. In areas where limited trained personnel are limited, it can be hard to get accurate numbers due to lack of staff and time to check for vital signs.
Detailed description of the solution[edit | edit source]
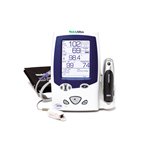
The Spot Vital Signs device measures the blood pressure, pulse, and temperature of patients accurately and rapidly. It is a portable device that runs on battery power. The device measures blood pressure in 15 seconds and is able to store data from previous measurements.
Designed by[edit | edit source]
- Designed by: Welch Allyn Inc.
- Manufacturer (if different): Welch Allyn Inc.
- Manufacturer location: Skaneateles Falls, New York, USA
Funding Source[edit | edit source]
The development of this device was supported by private funds.
References[edit | edit source]
Peer-reviewed publication[edit | edit source]
Alpert, B. S. (2007). Validation of the Welch Allyn Spot Vital Signs blood pressure device according to the ANSI/AAMI SP10: 2002. Accuracy and cost-efficiency successfully combined. Blood pressure monitoring, 12(5), 345-347.
Davis, P. D., Dennis, J. L., & Railton, R. (2005). Evaluation of the A&D UA-767 and Welch Allyn Spot Vital Signs noninvasive blood pressure monitors using a blood pressure simulator. Journal of Human Hypertension, 19, 197-203.
Other internally generated reports[edit | edit source]
Spot Vital Signs® LXi. (n.d.). Welch Allyn Medical Diagnostic Equipment Manufacturerwelchallyn.comwelchallyn.com. Retrieved November 10, 2012, from here.
IP and copyright[edit | edit source]
United States Patent & Trademark Office(USPTO) #7,780,603 filed in September 2005. Link available here.
Approval by regulatory bodies or standards boards[edit | edit source]
This device was FDA approved on July 9, 2010. Link available here.