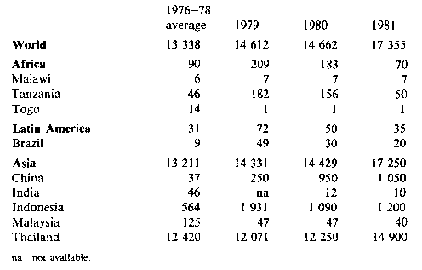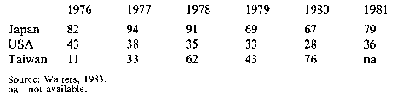CASSAVA, Manihot, Manioc, Tapioca, Tapioka.
Botanical name
Manihot esculenta Crantz syn. M. utilissima Pohl.
Family
Euphorbiaceae.
Other names
Aipi, Aipim ubi (Braz.); Bafifanapaka (Madag.); Brazilian arrowroot, Cassadal� (Afr.); Cassave (Nether.); Caxcamot� (Guat.); Cu san tau (Viet.); Delhazo (Madag.); Guacamote (Mex.); Kamoteng kahoy (Philipp.); Kasp� (Indon.); Kelala (Ind.); Ketalla (Braz.); Khoaimi (Viet.); Kute (agbeli) (Togo); Macaxeira, Mandioca (Braz.); Mandioko (Gam.); Manoco (P. Rico); Mayaca (Zar.); Obikajoe (Indon.); Ramu (Lat. Am.); San (Viet.); Tentu neskok (Philipp.); Ubi kayu (Mal.); Ubi singkong (Indon.); Yautia, Yuca (S. Am.).
Botany
Cassava is a perennial shrub, with latex in all its parts, which produces enlarged tuberous roots. There are over 100 cultivars and there is great variation in the form of the plant. The height ranges from about 1 to 3 m or more. The stems are usually slender and glabrous, with leaves borne near the apex; the lower parts of the stems have nodes made conspicuous by prominent leaf scars. Branching is variable; some cultivars branch near the base and are spreading in form, others are erect and branch nearer the apex. Stems vary in colour, being grey or silvery, green, greenish-yellow, reddish-brown, or streaked with purple. The leaves, which are spirally arranged with phyllotaxis 2/5, have petioles 5-30 cm long, usually longer than the blades; the blades are deeply palmately divided with 5-7 (occasionally 3-9) lobes, each 4-20 cm long and 1-6 cm wide, obovatelanceolate, pointed and with entire margins. They vary in colour from green to reddish; the petiole and midrib may be deep red. Older leaves are shed leaving the prominent leaf scars mentioned above. The flowers are borne in axillary racemes near the ends of branches, and are monoecious, pale yellow or red, 1-1.5 cm in diameter. The fruit is a six-winged capsule with 3 ellipsoidal seeds each about 12 mm long. Root tubers develop by a process of secondary thickening as swellings on adventitious roots a short distance from the stem.
Great variation is shown in the number, shape and size of the tubers and the angle at which they penetrate the ground. There are usually 5-10 tubers per plant, cylindrical or tapering, 3-15 cm in diameter and 15-100 cm long, occasionally longer. Hydrocyanic glycoside is present in varying quantity. Cassava clones are often classified by taste as 'sweet' or 'bitter', but - contrary to the commonly stated notion - this does not always reflect a direct relationship with the cyanogenic glycoside content of the root. The two types have sometimes been regarded as different species, the former being called M. esculenta and the latter M. palmata or M. dulcis. This division is clearly not tenable. Further, the toxicity of a cultivar varies according to environmental growth conditions. However, in any one location it is possible to find some cultivars 'bitter' and some 'sweet', so that a local separation between bitter and sweet can often be made.
Origin and distribution
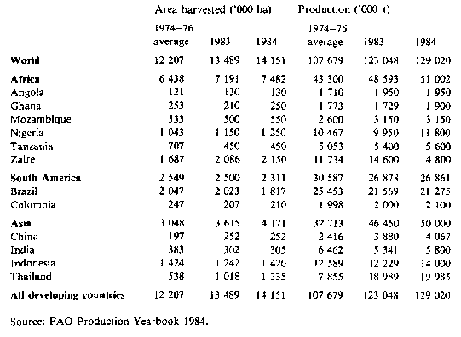
Manihot esculenta is not known in the wild state. Some 98 species of the genus Manihot have been found in the western hemisphere, and it appears that M. esculenta must have arisen by mutation or hybridisation, the probable centre being southern Mexico/Guatemala or north-eastern Brazil, or both. It was early domesticated and was cultivated in Peru 4 000 and in Mexico 2 000 years ago. It was subsequently spread throughout Central and tropical South America, and was taken by the Portuguese to Africa in the 16th century. Its spread in Africa was slow until the end of the 19th and first half of the 20th century, but Africa now produces almost 40 per cent of the world's total. The crop has become important throughout the tropics, under a wide range of conditions of climate and soil, with West Africa, Brazil, Indonesia and Thailand being major producers.
Cultivation conditions
Temperature - the optimum temperature range for cassava is 25-30°C, and the approximate boundaries for its culture are latitudes 30°N and 30°S. The minimum temperature for its growth is 18°C and yields are reduced above 30°C. It cannot withstand freezing and must have a minimum of 300 frost-free days.
Rainfall - a well-distributed annual rainfall of 100-150 cm is regarded as ideal, but the crop can be successfully grown in areas with rainfall ranging from 50 to 250 cm. Occasionally (eg in Kerala, India), with rainfall of less than 75 cm it is irrigated. Except at planting cassava can withstand prolonged periods of drought and is therefore a useful crop in areas of low or uncertain rainfall.
Soil - light sandy loams of medium fertility give the best results, but cultivars can be grown successfully on soils ranging from stiff marine clays with a pH of 8-9, to sands or loose laterites with a pH of 5-5.5. When grown on clay soils, the plant produces stem and leaf growth at the expense of the roots and many cultivars give poor yields. Saline and swampy soils are unsuitable. Cassava can tolerate soils of low fertility, especially if the feeder roots can penetrate to depths of 40-60 cm; deep cultivation before planting is therefore recommended.
Although it responds well to fertilisation, cassava will grow on relatively infertile soils which are unsuitable for other crops. However, it removes considerable quantities of nutrients from the soil; published figures indicate that for a crop of 25 t/ha of roots the following quantities of nutrients are absorbed: nitrogen 53.5 kg; phosphorus 26.3 kg; potassium 105 kg; calcium 17.2 kg and magnesium 9.75 kg. Thus continuous growing of cassava on improverished soils leads to even more severe soil deficiencies. Potassium is often the limiting factor. Proper fertilising practice will depend upon the soil in question (and on other limiting factors, such as rainfall) but a general basic recommendation is to use about 500 kg/ha of a 12:12:18 complete (NPK) fertiliser. FYM at 20-30 t/ha augmented with 500 kg/ha of rock phosphate and 300 kg/ha of muriate of potash has given good results in Madagascar with average yield of roots about 40 t/ha.
Altitude - cassava can be grown from sea level up to about 1 000 m in equatorial regions, though at the highest altitudes growth is slow and yields are reduced.
Day-length - cassava is a short-day plant and less productive of tuberous roots in day-lengths greater than 10-12 hours; it is, therefore, most productive when grown in areas between latitudes 15°N and 15°S, though some cultivars will tolerate longer days and extend the limits to 30°N and 30°S.
Planting procedure
Material - seed is difficult to germinate and is used only for breeding work. In any case it is necessary to use vegetative material to propagate cultivars and stem cuttings (sticks) are used. For hand planting, sticks about 20-30 cm long, 2.5-3.75 cm thick and with at least 5 buds, taken from stems 8-18 months old are recommended. It is essential that virus-free material be selected and treatment of the cuttings with a mixture of fungicides and insecticides (eg maneb + propineb + copper oxychloride + malathion) has been advised. The sticks can be stored for up to 8 weeks in cool, well-ventilated conditions except when harvested under rainy conditions, when storage is normally reduced to 7-10 days. For mechanical planting shorter sticks, 15-20 cm long, are used.
Method - most cassava is still planted by hand, though mechanisation is increasing. Planting is normally at the start of the rainy season, often in flat fields, though planting on ridges is desirable in wet regions. The sticks may be cut obliquely or at right angles to the axis, and planted vertically or at an angle, with half their length in the soil, or flat below the surface at a depth of about 10 cm. When sticks are planted vertically or inclined, tubers form only at the extreme end of the cut, forming a slanted cluster often of irregular size; when a stick is cut at right angles and planted vertically, the roots are evenly distributed around the circumference and are more uniform in size. Horizontally-planted sticks produce tubers at each node, but lodging of the aerial part is increased and yields reduced. With vertical or inclined planting the roots penetrate more deeply and tubers may be formed at intervals along the planted portion, but in areas of low rainfall desiccation of the cuttings may occur. After 8-12 weeks the plants are usually earthed up to encourage tuber formation.
In smallholdings cassava is frequently grown in mixed cultivations, eg among vegetables, bananas, yams or sweet potatoes, or intercropped with rubber and coconuts: it has been shown that financial gains can be made by certain mixed cultivation systems when compared with monoculture of cassava. When interplanted with coconuts a ground cover of the legume Stylosanthes sp. (stylo) has led to a substantial increase in yield.
Mechanisation of planting (and of the complete production system) is practiced on a large scale in Brazil, parts of Africa and elsewhere. Several types of machine have been developed or modified from vegetable planters: some plant the cuttings vertically or at an angle, others plant the cuttings horizontally in a trench which the machine then covers. The rate of planting can be up to 3-4 ha/day. Sprouting usually takes place in about a week with only about a 5 per cent failure rate: new cuttings are substituted within a month.
Weed control is extremely important, the timing and frequency depending upon local conditions, but, assuming planting was in a clean field, the first weeding should normally be 2-3 weeks after sprouting, followed by three or four weedings during the following 4-6 months. Chemical control gives excellent results: pre-emergence application of, for example, linuron or diuron with subsequent application of shielded sprays of paraquat, has proved successful.
Field spacing - the plant density preferred varies greatly from country to country and within countries, and is affected by cultivar, soil conditions, local customs, and the use to which the roots will be put: a range of between 3 000 and 20 000 plants/ha is quoted for cassava for direct eating (the closer spacing is used on the more infertile soils) but 7 000 to 10 000 plants/ha is the more usual range. If other factors remain constant, closer spacing gives higher yields but smaller roots, and is thus favoured for mechanical harvesting of tubers to be used for processing. In parts of Brazil, with certain cultivars, 30 000 plants/ha are used. High densities of planting, however, tend to encourage certain fungal diseases.
Seed rate - planting is usually at 90 cm intervals in rows 90-120 cm apart. With tows 120 cm apart and 90 cm spacing along the row, the plant density would be approximately 9,250/ha.
Pests and diseases
Pests - root knot nematodes (Meloidogyne spp.) are common but seldom of economic importance except when cassava is intercropped with good hosts for the nematode, eg egg plant (Solanum melongena) or Hibiscus sabdariffa. Pratylenchus sp., Helicotylenchus erythrinae, Rotylenchus reniformis and a few other species have been found in cassava but are considered of little importance.
A number of insects may cause defoliation. In Africa the grasshopper Zonocerus variegatus may be serious; control is by destruction of the egglaying sites or by fenitrothion. Cassava hornworm (Erinnyis ello) may attack the crop in the Americas and Caribbean; biological control is best, by Bacillus thuringiensis sprays, by the egg parasite Trichogromma fasciatum, or by the predatory wasp Polistes canadensis. Leaf cutting ants, Atta sp. and Acromyrmex sp., can cause severe defoliation; poisoned bait is the recommended control. In South America the cassava lacebug (Vatiga manihotae) may cause early defoliation; fenitrothion will control the pest, but will not be economic unless the attack is severe. Among borers in South America, the shootflies, Silba pendula and Carpolonchaea chalybea, burrow into the growing point at the start of the rainy season; resistant cultivars are recommended. Control of larvae may be with diazinon, dimethoate or other chemicals. Stems may be attacked by the weevil borers, Coelosternus spp.; cutting off the infected branches is recommended for control. Scale insects are reported to cause damage especially in areas subject to drought. These include the cassava stem mussel scale (Aonidomytilus albus), the white peach scale (Pseudaulacaspis pentagona), and the black scale (Parasaissetia nigra); oil emulsion plus malathion is recommended for control. Spider mites, Mononychellus tanajoa and Tetranychus cinnabarinus, sometimes affect the crop; mitetolerant cultivars have been developed and M. tanajoa is attacked by predaceous mites and staphylinid beetles, so that chemical control should not be necessary. However, control can be obtained by a number of acaricides. In some areas rodents, baboons, monkeys and wild pigs are reported to cause severe damage, and smallholders sometimes plant bitter cassava around their areas of sweet cassava to discourage such predators.
Diseases - cassava is subject to several virus diseases. In Africa, the most important is African mosaic or curly leaf disease (manihot virus), transmitted by a white fly (Bemisia sp.). No host plant is known for this virus. A virus producing similar effects, Asian mosaic, occurs in India; Cucumis sativus (cucumber) is reported to be a host. In northern South America another mosaic, also causing chlorosis and leaf curl, has several hosts, eg other species of Manihot, some Chenopodiaceae and some Malvaceae. Recommended control measures include the removal and destruction of diseased plants, the use of healthy planting material of resistant cultivars, and the control of vectors and host plants where these are known.
Of the fungal diseases the most important are leaf spots caused by Cercospora henningsii and C. caribaea. These may be controlled by copper fungicides, thiophanate or benomyl, but chemical methods are unlikely to be economic and the use of resistant cultivars is recommended, and measures to reduce the humidity in the stand, eg wider spacing. Phyllosticra sp. also causes leaf-spotting; no specific control has been reported except the possible use of resistant material. Uromyces spp. cause rusts of leaves and stems but do not appear to be of serious economic importance. Stem rots on stored planting material can cause loss of viability. Glomerella sp., Botryodiplodia theobromae and some Basidiomycetes and Ascomycetes are causative agents. Control involves careful selection and handling to avoid wounding, and storage under not excessively humid conditions. 'Seed treatment' fungicides, eg captan+carbendazim, mancozeb and chloroneb, are among those recommended. Root rots include those caused by Phytophthora spp., Rigidoporus lignosus (white rot, white thread), Rosellinia necatrix (black rot), Corticium rolfsii, and a number of others of lesser importance. Control is by cultural practices, including drainage, early harvest, crop rotation, the removal of crop debris, and planting of healthy material. The most important bacterial disease is cassava bacterial blight, caused by Xanthosoma manihotis, which appears as leaf spotting and blight, wilting, die-back, gum exudation and vascular necrosis throughout the plant; resistant cultivars are available. Other bacterial diseases, but of less importance, are bacterial stem rot (Erwinia cassavae) and Agrobacterium sp. (bacterial stem gall).
A package of integrated pest control has been advocated for cassava. The first objective is to ensure healthy and resistant plants by good soil preparation and good drainage, the use of robust sticks taken from well-lignified stems, free from disease and undamaged, dipped in fungicide (captan + benomyl) before planting, correct planting practices, including wide spacing between plants, good weed control and fertilising. Weed control is important not only to avoid all competition with the crop, but also to avoid the appearance of plants that may be hosts to cassava pests. Disease- and pest-resistant cultivars should be used where possible. As monoculture is the usual practice different cultivars should be planted together or in adjacent plots, to provide different degrees of susceptibility (or resistance) within the planted areas. Regular inspections should be made so that appropriate pest control may be applied at an early stage when necessary. Natural biological control is important and chemicals should be used only when absolutely necessary. After harvest all plant residues should be removed. Continuous planting of adjacent areas should be avoided as this gives the opportunity for pests to have uninterrupted access to plants at their most susceptible age.
Growth period
Generally cassava reaches maturity in 9-24 months, according to the cultivar, climate and soil conditions. A few quick-growing cultivars can be harvested in 6-7 months, but good yields are normally only obtained after 9-12 months. When used as a vegetable the tubers are normally harvested within 12 months, otherwise they become very fibrous.
Harvesting and handling
The exact time, in terms of months after planting, when the tubers are ready for harvesting depends very much on the cultivar and growth conditions. Delays in harvesting do not seriously affect tuber quality or yield. The plants are normally topped, ie the above ground parts removed by hand, using a machete, or by machine, eg pushed down by a heavy screen mounted on the front of a tractor. The roots may then be dug by hand, but machine harvesting is being increasingly employed. A number of devices are used, from simple 'ridge breakers' which expose the roots and leave them to be picked up by hand, to relatively sophisticated equipment somewhat resembling potato harvesters. Damage to tubers is still a problem with mechanical harvesting, but smaller tubers are more easily lifted and less liable to damage. For this reason mechanical harvesting is particularly suitable for tubers to be used for processing, where small tubers are satisfactory, and a greater degree of damage can be tolerated.
After harvesting, cassava tubers deteriorate rapidly. There are two distinct types of deterioration which occur during storage, one physiological and the other due to microorganisms. Physiological deterioration, which begins to appear within three days, is essentially a humidity-sensitive wound response with increases in enzyme activity leading to the production of phenols including catechins and leucoanthocyanidins which in the later stages of discoloration polymerise to form condensed tannins. Visible signs of discoloration are at first blue, becoming brown, and initially generally appear in the peripheral vascular bundles and spread to adjacent parenchyma. Physiological deterioration appears to be connected with enzyme activity and some measure of control has been obtained by holding the roots at low temperature, or storing in air with low oxygen levels or in carbon dioxide. Mechanical damage to the roots also permits the entry of microorganisms, causing rapidly-spreading internal rotting. Storage under conditions that favour wound healing, such as packing in moisture-absorbent material, has been reported to minimise physiological deterioration and invasion by pathogens to give a storage life of 4 or more weeks. Pre-pruning of aerial portions of the plant 2-3 weeks before harvesting has also been shown to minimise physiological deterioration in the tubers Coating with a fungicidal wax is stated to extend the storage life up to 1-2 months under ambient conditions in the tropics, while holding undamaged tubers at 0-2°C and 85-90 per cent RH is reported as satisfactory for periods of about 4 weeks.
Primary product
Tubers - these are dark coloured, fleshy and cylindrical, varying a great deal in size and form, with a starch content of 20-40 per cent. Each plant normally yields 5-10 tubers, usually 30-45 cm long, with a diameter of 5-15 cm and weighing 0.9-2.3 kg. The peel accounts for 10-20 per cent of the tuber and consists of an outer corky rind and an inner part which separates the peel from the flesh of the roots.
Yield
Yields vary greatly according to cultivar, soil, climate, age at harvesting, etc. Average yields in t/ha for 1984 were quoted as follows: world 9.1; Central America and the Caribbean 5.6; Africa 6.8; Oceania 10.7; South America 11.6; Asia 12. Of the major producers Thailand had the highest average yield, 15 t/ha, though a very minor producer, the Cook Islands, is reported to have averaged 32.5 t/ha. In fact average yields on a world basis changed little during the decade 1974-84; that for 1974-76 was 8.8 t/ha and for 1984 the figure was 9.1. Yields of smallholdings normally range from 5 to 15 t/ha under normal conditions but can drop to 3 t/ha on poor soils without fertilisation. On plantations yields of 30-40 t/ha are normal and with selected high-yielding cultivars can exceed 50 t/ha. Under poor soil conditions appropriate fertilising can triple or quadruple yields.
Main use
Cassava is the staple food of the poorer section of the population of many tropical countries, and has been estimated to provide 37, 12 and 7 per cent of the energy in the diet of the tropical areas of Africa, America and Asia respectively. The fresh peeled tubers are eaten as a vegetable after boiling or roasting. They are often boiled and pounded into a paste and added to soups and stews ('fufu' in Nigeria). As the fresh tubers deteriorate rapidly they are often preserved in the form of sun-dried chips ('kokonte' in West Africa) and consumed after cooking or being ground into a flour. The principal form in which cassava is eaten in West Africa is as a fermented meal known as 'gari', while in Central and South America a product, 'farinha de manioca' which is similar to 'gari' except that much less fermentation occurs during its preparation, is very popular. In the Philippines 'landang' or 'cassava rice' is prepared and retains much of the original small quantity of protein.
Subsidiary uses
Starch - a substantial industrial outlet for cassava is in the manufacture of starch for use in the foodstuff, textile and paper industries, the manufacture of plywood and veneer, adhesives, glucose and dextrin. Minor industrial applications include use in the manufacture of explosives, dyes, drugs, chemicals, carpets and linoleum, the production of alcohol and the coagulation of rubber latex.
Dried cassava roots - increasing quantities of dried cassava roots are being used for livestock feeding, particularly in the EC countries. Formerly, they entered international trade in the form of chips, made by slicing the roots and then sun drying, but these have almost completely been supplanted by pellets made by grinding the dried chips and compressing the powder into portions approximately 2 cm long and I cm in diameter. These pellets are used as a carbohydrate source in animal feed rations, particularly for pigs
Tapioca - made from cassava starch and used for the preparation of puddings and in infant and invalid foods. In Thailand tapioca is known as sago, which can lead to confusion with true sago starch obtained from the sago palm, Metroxylon sagu.
Cassava flour - the flour is used in the preparation of bread, biscuits and confectionery and in products such as macaroni, spaghetti and rice substitutes, also as an adulterant of cereal flour and in the production of adhesives.
Glucose - which is produced from cassava in Kerala (southern India).
Secondary and waste products
Cassava meal - about 10-20 per cent residue is left after the extraction of starch and tapioca, and this can be used as a livestock feed, or as a raw material for the production of adhesives. The approximate composition (dry matter) is: protein 5.3 per cent; fat 0.1 per cent; starch 56 per cent; fibre 35.9 per cent; ash 2.7 per cent.
Leaves - young leaves are eaten in some areas of Africa as a vegetable. Mature leaves, which have a protein content of 5-7 per cent, can be used for animal feeding and are sometimes dried and ground into a meal. They have a high lysine content.
Stems - the possibility of utilising cassava stems for the manufacture of particle board has recently been investigated.
Juice - the juice expressed from the tubers during starch production is sometimes concentrated and spices added to obtain a sauce, known as 'cassari po' or 'cassareep' in the West Indies and 'tucupi' in Brazil.
Miscellaneous - the tubers may be stored as silage and used for animal feeding. In some countries fermented beers are prepared on a small scale. In Brazil, alcohol has been produced directly from cassava roots, through malt saccharification and immediate fermentation, but cane sugar alcohol can now be produced more cheaply.
Special features
The typical range of composition for the edible portion of the tubers is: energy 607 kJ/100 g; water 62-65 per cent; protein 0.7-2.6 per cent; fat 0.2-0.5 per cent; total carbohydrate 32-35 per cent; fibre 0.8-1.3 per cent; ash 0.3-1.3 per cent; calcium 33 mg/100 g; iron 0.7 mg/100 g; thiamine 0.06 mg/100 g; riboflavin 0.03 mg/lOOg; niacin 0.6 mg/100 g; ascorbic acid 20-30 mg/100 g; vitamin B 10 IU/100 g.
The principal amino acids present in the protein are arginine, histidine, isoleucine, leucine and Iysine. Owing to the low protein content the disease Kwashiorkor is prevalent in areas where cassava is the main staple item of diet. A method of enriching the protein by innoculation of cassava flour paste with Rhizopus or other suitable fungi has recently been developed and the protein content can be increased to about 3.25 per cent.
Cassava roots contain the glycoside linamarin which is converted into HCN by the enzyme linamarase. HCN contents can vary from 10 to 490 mg/kg, being highest in roots grown on soils of low fertility, particularly if there is a potassium deficiency, and also in the first year's growth and in the dry season. It is usually highest in the rind and in the fibrous core at the centre, but considerable variation can occur between various parts of the same tuber. For edible purposes cultivars with a high starch and protein content and a low HCN content are preferred. For starch manufacture cultivars with a high starch content are favoured and HCN content is of less importance. As all cultivars contain some cyanogenic glycoside all are toxic in some degree and 'chronic cassava toxicity' is recognised in populations in which cassava is a major portion of the diet, particularly in Africa, as goitre and tropical ataxic neuropathy. In severe cases cassava can cause respiratory difficulties and occasionally death. Detoxification of bitter cassava is normally practiced. Peeling and cooking gives a partial detoxification, but soaking of roots for long periods, repeated boiling in changes of water, soaking after comminution, and fermentation or fermentation followed by heat treatment, are all used. However, there appears to be no evidence that animals develop toxicity symptoms from continuous intake of cassava or cassava forage.
Cassava starch granules are of various shapes, round, truncated, etc, and vary in size from 5 to 35 microns (average 15-17 microns). The amylose content is about 17 per cent compared with 22 per cent for potato starch and 27 per cent for maize starch. The approximate composition of commercial samples is: moisture 9-18 per cent; protein 0.31-1 per cent; fat 0.1-0.4 per cent; starch (and a little fibre, etc) 81-89 per cent; ash 0.1-0.8 per cent. Good quality starch should be absolutely free from specks and have a pure white colour, a pH of 4.7-5.3, a moisture content of 10-13.5 per cent, and an ash content of less than 0.2 per cent.
Processing
Starch production
(i) The mature roots are first washed to remove dirt and loose soil.
(ii) In small-scale operations the roots are then peeled by hand to remove the skin and cortex; on a factory scale only the outer skin is removed, since when processing large quantities of roots it becomes economic to recover the starch from the cortex although it only contains about 50 per cent of that in the core of the root.
(iii) The roots are next sliced and put through a rasping or grating machine to produce a slurry or pulp.
(iv) The slurry is then sieved to separate the fibrous tissue from the starch milk; considerable quantities of clean water are used at this stage in order to ensure efficient separation of the starch granules from the slurry.
(v) The starch milk is collected and left in settling tanks for at least 6 hours, when the starch sinks to the bottom and the liquid is drained away.
(vi) The surface layer of the starch mass is usually a yellowish-green colour and contains impurities and is therefore scraped off, leaving a creamy-white mass below, which is stirred vigorously with water and then left to settle. This washing and settling process is repeated once or twice more until the starch is judged to be sufficiently pure.
(vii) The starch cake is dried, either by spreading it out in trays in the sun or in factories in hot-air driers.
(viii) Finally, the hard lumps of starch are crushed into a powder and sieved.
It should be pointed out that, because the cells of cassava roots are relatively tough, the grinding process must be efficient in order to liberate all the starch granules and to obtain a commercial extraction rate of approximately 20-25 per cent of the raw material.
Tapioca - tapioca consists of pieces of partially-gelatinised cassava starch and can be prepared in the form of flakes, seeds and pearls. In the preparation of tapioca flakes, the moist starch, prepared as above, is rubbed through a sieve of about 8 mesh/cm to give a coarse ground moist flour, and partially gelatinised by cooking for about 2 minutes in iron pans previously smeared with oil. The flakes of tapioca are then dried at about 50°C to a moisture content of approximately 12 per cent. In the preparation of tapioca seeds or pearls, the sieved damp starch is made into globules by shaking in cloth bags or by the use of mechanically-operated granulators. The globules are then graded according to size and gelatinised by roasting them for about 15 minutes in hot pans smeared with coconut oil. They are finally dried in a hot-air drier at 40-50°C for about 1.5-2 hours; the yield of tapioca from fresh tubers is usually about 25 per cent.
Production and trade
Production - world output increased on average 2 per cent/a for the period 1974-84, reaching nearly 129 million tonnes of roots (corresponding to about 46 million tonnes of grain equivalent). The largest increases were in Laos, Thailand, the Philippines and Vietnam, but Brazil showed a decline. A slight fall in 1982 was mainly the result of lower production from Thailand. In Africa, supplies still fell short of the food requirements of the population. In the Far East consumption increased in China, Indonesia and the Philippines. World wide, about 65 per cent of the production is consumed direct as human food and about 30 per cent is consumed or processed as animal feed, of which about half is exported (see next section). About 4 per cent is converted into starch and other industrial products and rather less than I per cent into ethanol, mostly in Brazil.
For production data in the more important producing countries see Table 1.
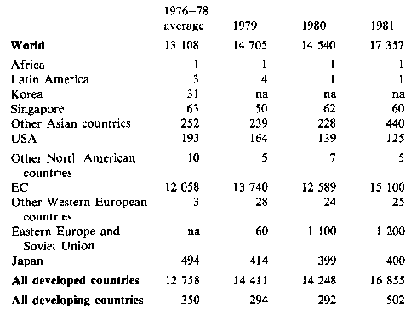
Trade - because fresh cassava deteriorates rapidly only a very small quantity of fresh roots is traded internationally, mainly to immigrant populations. However, substantial quantities of processed roots (chips and pellets) and cassava starch are traded; world trade in 1981 is estimated to have exceeded 17 million tonnes in root equivalent, compared with 14.7 million tonnes in 1980. Tables 2 and 3 show gross imports and exports for the major cassava trading countries. Exports from China, Indonesia and Thailand rose substantially, with Thailand providing nearly all the 5.5 million tonnes to the EC permitted by a 'voluntary' agreement which reduces this quota to 5 million tonnes for 1983 and 1984, and 4.5 million tonnes for 1985 and 1986. With present EC policies, the market for cassava as animal feed seems unlikely to develop further, nor does there appear to be much likelihood of increased demand elsewhere: 1981 may represent a peak year for trade in this commodity, with relative stability or a slight decline in the forseeable future.
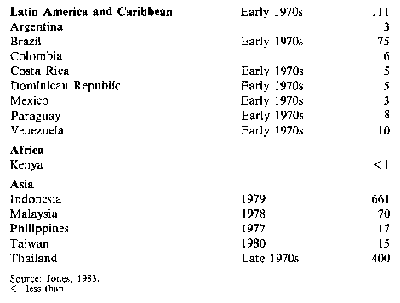
Only about 4 per cent of world starch production moves in trade and most of this is cassava starch: the major importers are Japan, the USA and Taiwan. However, in the USA home-produced maize starch is taking the place of cassava starch and in Japan the domestic starch industry is highly protected by quotas and tariffs, with the result that there has been a decline in imports of cassava starch in these countries. Only in Taiwan has there been an increase. Table 4 shows production of cassava starch and
Table 5 imports by by the importing countries It is believed that in developed countries the decline in demand will continue and that while, for a time, demand in developing countries may increase, locally-produced starches will eventually reduce imports.
Table 1: Cassava: Area and production in selected countries
Table 2: Cassava: Exports from selected countries ('000 t root equivalent)
Table 3: Cassava: Imports by selected countries ('000 t root equivalent)
North America and Japan take most of their cassava imports in the form of starch and tapioca; pellets for animal feed predominate in the European and Soviet Union imports. na not available.
Table 4: Cassava starch: Production in selected areas ('000 t)
Table 5: Cassava starch: Imports to major markets ('000 t)
Major influences
Cassava produces more starch per hectare under relatively dry conditions than any other known crop. Production is likely to increase in semi-arid areas of the tropics, especially where the land is level and machinery can be used, and it may replace to some extent yams, aroids and sweet potatoes in local diets. Increasing urbanisation in the tropics is likely to lead to an increase in plantation production particularly where there is a need to supply local processing units. This will result in the increasing mechanisation of crop production, although mechanical harvesting still poses problems.
The overall prospects for any significant increase in international trade in cassava products is limited at present price levels. Any future increase in cassava production will therefore need to be utilised primarily in the producing countries themselves.
Bibliography
ACENA, B. and PUNO, G. D. 1955. A study of the use of cassava in the beer industry. Philippine Journal of Agriculture, 20, 1-13.
ADRIAN, J. and PEYROT, F. 1971. Possible use of the cassava leaf (Manihot utilissima) in human nutrition. Qualitas Plantarum: Plant Foods for Human Nutrition, 2 (2), 61-65.
AKINRELE, I. A. 1967. Nutrient enrichment of gari. West African Journal of Biological and Applied Chemistry, 10 (1), 19-23.
ANON. 1972. The mechanised production of gari. Food Manufacture, 47, 62-63.
ARRAUDEAU, M. 1967. Cassava in the Malagasy Republic. Proceedings of the International Symposium on Tropical Root Crops (Trinidad, 1967) (Tai, E. A., Charles, W. B., Haynes, P. H., Iton, E. F. and Leslie, K. A., eds), Vol. 1, Section 111, pp. 180-184. St. Augustine, Trinidad: University of the West Indies (2 vole).
AYRES, J. C. 1972. Manioc. Food Technology, 26 (4), 128-132; 134; 136; 138.
BALAGOPAL, C., VIJAYAGOPAL, K. and HRISHI, N. 1982. Bioconversion of cassava - a potential source of energy in India. Proceedings of the 5th International Symposium on Tropical Root and Tuber Crops (Philippines, 1979), pp. 339-344. Los Ba�os, Laguna, Philippines: Philippine Council for Agriculture and Resources Research, 720 pp.
BEENY, J. M. 1969. Mechanisation for tapioca. Incorporated Society of Planters, Proceedings of Conference on Crop Diversification (Malaysia) (Blencowe, E. K. and Blencowe, J. W., eds), pp. 167-180. Kuala Lumpur, Malaysia: Yau Seng Press, 300 pp.
BELLOTTI, A. C., REYES, J. A., ARIAS, B. and VARGAS, O. 1982. Insectos y acaros de la yuca y su control. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E., ed.), pp. 367-391. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
BELLOTTI, A. C., VARGAS, O., PE�A, J. E. and ARIAS, B. 1982. Perdidas en rendimiento en yuca causadas por insectos y acaros. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E., ed.), pp. 393-408. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
BEST, R. and GOMEZ, G. 1982. Procesamiento de las raices de yuca pare alimentaci�n animal. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E. ed.), pp. 513-538. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
BOLHUIS, G. G. 1966. influence of length of illumination period on root formation in cassava. Netherlands Journal of Agricultural Science, 14, 251-254.
BRAMBILLA, J., HELFGOTT, S. and RUIZ, V. 1983. Integrated weed control in cassava. Abstracts of the 6th Symposium of the International Society for Tropical Root Crops (Peru, 1983), p. 24. Lima, Peru: International Potato Center, 113 pp.
BROOK, E. J., STANTON, W. R. and WALLBRIDGE, A. 1969. Fermentation methods for protein enrichment of cassava. Biotechnology and Bioengineering, I I, 1271 - 1284.
BRUIJN, G. H. de. 1971. �tude de caract�re cyanog�n�tique du manioc (Manihot esculenta Crantz). [A study on the cyanogenetic character of cassava.] Thesis with English summary, pp. 124-128. Mendelingen Landbouwhogeschool, Wageningen, 71-13. Wageningen, Netherlands: H. Veenman and Zonen, N. V., 140 pp.
CARIBBEAN FOOD AND NUTRITION INSTITUTE. 1974. Food composition tables for use in the English-speaking Caribbean. Kingston, Jamaica: CFNI, 115 pp.
CEDILLO, V. G. 1952. Cassava rice or landang. Philippine Agriculturist, 35, 434-440.
CENTRE FOR OVERSEAS PEST RESEARCH. 1978. Pest control in tropical root crops. PANS Manual, No. 4. London: COPR, 235 pp.
CHADHA, Y. R. 1961. Sources of starch in Commonwealth territories. III: Cassava. Tropical Science, 3, 101-113.
CHAN SEAK KHEN. 1969. Notes on the growing of cassava at Serdang. Incorporated Society of Planters, Proceedings of Conference on Crop Diversification (Malaysia) (Blencowe, E. K. and Blencowe, J. W., eds), pp. 139-148. Kuala Lumpur, Malaysia: Yau Seng Press, 300 pp.
COCK, J. H., CASTRO, M. A. and CESAR TORO, J. 1978. Agronomic implications of mechanical harvesting. Cassava Harvesting and Processing: Proceedings of a workshop held at CIAT (Colombia, 1978), IDRC-114e (Weber, E. J., Cock, J. H. and Chouinard, A., eds), pp. 60-65. Ottawa, Canada: International Development Research Centre, 84 pp.
COOKE, R. D. and COURSEY, D. G. 1981. Cassava: a major cyanide-containing food crop. Cyanide in biology (Vennesland, B., Conn, E. E., Knowles, C. J., West, J. and Wissing, F., eds), pp. 93-114. New York: Academic Press, 548 pp.
COURSEY, D. G. and HAYNES, P. H. 1970. Root crops and their potential as food in the tropics. World Crops, 22, 261-265.
DINA, J. A. AND AKINRELE, I. A. 1970. An economic feasibility study for the establishment of a glucose industry in Nigeria. Federal Institute of Industrial Research, Nigeria, Technical Memorandum, No. 25, 28 pp.
DOKU, E. V. 1969. Cassava in Ghana. Accra, Ghana: Universities Press, 44 pp.
DOLL, J. D., PIEDRAHITA, C. and LEIHNER, D. E. 1982. Metodos de control de malezas en yuca (Manihot esculenta Crantz). Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E., ed.), pp. 241-249. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
FLAWS, L. J. and PALMER, E. R. 1968. The production of particle board from cassava stalks. Report of the Tropical Products Institute, G34. London: TPI, 3 pp.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS. 1982. Cassava. Commodity Review and Outlook 1982, pp. 52-55. Rome, Italy: FAO, 128 pp.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS. 1983. Cassava. Production Yearbook 1982, pp. 128-129. Rome: FAO, 320 pp.
FR�HLICH, G. and RODEWALD, W. 1970. Cassava. Pests and diseases of tropical crops and their control, pp. 173-176. Oxford: Pergamon Press, 371 pp.
GHOSH, B. N. 1967. Recent developments in the manufacture of starch from cassava roots in Uganda. Proceedings of the International Symposium on Tropical Root Crops (Trinidad, 1967) (Tai, E. A., Charles, W. B., Haynes, P. H., Iton, E. F. and Leslie, K. A., eds), Vol. 2, Section VI, pp. 37-47. St. Augustine, Trinidad: University of the West Indies (2 vole).
G�MEZ, G. G., CAMACHO, C. AND MANER, J. H. 1977. Utilization of cassava-based diets in swine feeding. Proceedings of the 4th Symposium of the International Society for Tropical Root Crops (Colombia, 1976), IDRC-080e (Cock, J., Maclntyre, R. and Graham, M., eds), pp. 262-266. Ottawa, Canada: International Development Research Centre, 277 pp.
GRACE, M. 1971. Processing of cassava. Food and Agriculture Organization of the United Nations Agricultural Services Bulletin, No. 8. Rome, Italy: FAO, 124 pp.
HAMID, K. and JALALUDIN, S. 1972. The utilisation of tapioca in rations for laying poultry. Malaysian Agricultural Research, I (1), 48-53.
HARRIS, N. V. and DOGGRELL, L. M. 1984. Developing cassava as an industrial crop in Australia. (Abstract). Proceedings of the 6th Symposium of the International Society for Tropical Root Crops (Peru, 1983), p. 431. Lima, Peru: International Potato Center, 672 pp.
HORTON, D., LYNAM, J. AND KNIPSCHEER, H. 1984. Root crops in developing countries - an economic appraisal. Proceedings of the 6th Symposium of the International Society for Tropical Root Crops (Peru, 1983), pp. 9-39. Lima, Peru: International Potato Center, 672 pp.
HOWELER, R. H. 1982. Nutrici�n mineral y fertilisaci�n de la yuca. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C.E., ed.), pp. 317-357. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
INGRAM, J. S. 1970. Selected bibliography on cassava (Manihot esculenta Crantz). Report of the Tropical Products Institute, G51. London: TPI, 35 pp.
INGRAM, J. S. and HUMPHRIES, J. R. O. 1972. Cassava storage: a review. Tropical Science, 14, 131-148.
JACOB, A. and UEXKULL, H von. 1960. Cassava. Fertiliser use: Nutrition and manuring of tropical crops, pp. 151-157. Hannover, Germany: Verlagsgesellschaft f�r Acherbau mbH, 617 pp.
JARMAI, S. 1968. A new fast method for the production of kokonte. Ghana Journal of Agricultural Science, I (1), 59-63.
JENNINGS, D. L. 1970. Cassava in Africa. Field Crop Abstracts, 23, 271 -278.
JOHNSON, R. M. and RAYMOND, W. D. 1965. The chemical composition of some tropical food plants. IV: Manioc. Tropical Science, 7, 109-115.
JONES, S. F. 1983. The world market for starch and starch products with particular reference to cassava (tapioca) starch. Report of the Tropical Development and Research Institute, G173. London: TDRI, viii+98 pp.
JONES, W. O. 1959. Manioc in Africa. Stanford, California: Stanford University Press, 315 pp.
KASASIAN, L. 1971. Root crops: Manihot esculenta (cassava, manioc). Weed control in the tropics, p. 159. London: Leonard Hill, 307 pp.
KOENS, A. J. and BOLHUIS, G. G. 1948. Knolgewassen: cassava. De Landbouw in den Indische Archipel. [Agriculture in the Indonesian Archipelago.] (Hall, C. J. J. van and Koppel, C. van de, eds), Vol. 2a, pp. 166-200. The Hague, Netherlands: NVUW van Hoeve, 905 pp.
KROCHMAL, A. 1966. Labour input and mechanisation of cassava. World Crops, 18 (3), 28-30.
KROCHMAL, A. 1969. Propagation of cassava. World Crops, 21, 193-195.
LEE KOK CHOO, T. and HUTAGALUNG, R. I. 1972. Nutritional value of tapioca leaf (Manihot utilissima) for swine. Malaysian Agricultural Research, 1(1), 38-47.
LEIHNER, D. E., THUNG, H., COCK, J. H. and LYMAN, J. K. 1982. Cosecha mechanica de la yuca, dos equipos diferentes. Yuca, Investigaci�n, Producci�n y Utilisaci�n, (Dominguez, C. E., ed.), pp. 359-364. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
LEIHNER, D. E., THUNG, H., COCK, J. H. AND LYMAN, J. K. 1982. Producci�n de yuca in cultivos multiples. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E., ed.), pp. 261-315. Cali, Colombia: Centro International de Agricultura Tropical, 659 pp.
L�ON, J. 1977. Origin, evolution and early dispersal of root and tuber crops. Proceedings of the 4th Symposium of the International Society for Tropical Root Crops (Colombia, 1976), IDRC-080e (Cock, J., MacIntyre, R. and Graham, M., eds), pp. 20-36. Ottawa, Canada: International Development Research Centre, 277 pp.
LOZANO, J. C. and BELLOTTI, A. C. 1982. Control integrado de enfermedades y pestes en la yuca. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E" ed.), pp. 463-473. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
LOZANO, J. C. and BOOTH, R. H. 1982. Enfermedades de la yuca. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E., ed.), pp. 421-461. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
LOZANO, J. C., HERSHEY, C. H., BELLOTTI, A. and ZEIGLER, R. 1984. A comprehensive breeding approach to pest and disease problems of cassava. Proceedings of the 6th Symposium of the International Society for Tropical Root Crops (Peru, 1983), pp. 315-320. Lima, Peru: International Potato Center, 672 pp.
LOZANO, J. C., TORO, J. C., CASTRO, A. and BELLOTTI, A. C. 1982. Selecci�n y preparaci�n de estacas de yuca pare siembra. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E., ed.), pp. 209-229. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
LULOFS, R. B. 1969. A study of methods and costs for commercial planting of tapioca in Kedah. Incorporated Society of Planters, Proceedings of Conference on Crop Diversification (Malaysia) (Blencowe, E. K. and Blencowe, J. W., eds), pp. 149-166. Kuala Lumpur, Malaysia: Yau Seng Press, 300 pp.
MAHENDRANATHAN, T. 1971. Potential of tapioca (Manihot utilissima Pohl) as a livestock feed - a review. Malaysian Agricultural Journal, 48 (1), 77-89.
MANER, J. N., BUITRAGO, J. and JIMENEZ, I. 1967. Utilization of yuca in swine feeding. Proceedings of the International Symposium on Tropical Root Crops (Trinidad, 1967) (Tai, E. A., Charles, W. B., Haynes, P. H., Iton, E. F. and Leslie, K. A., eds), Vol. 2, Section VI, pp. 62-71. St. Augustine, Trinidad: University of the West Indies (2 vole).
MARTIN, F. W. 1970. Cassava in the world of tomorrow. Tropical Root and Tuber Crops Tomorrow: Proceedings of the 2nd International Symposium on Tropical Root and Tuber Crops (Hawaii, 1970) (Plucknett, D. L., ed.), Vol. I, pp. 53-58. Honolulu, Hawaii: College of Tropical Agriculture, University of Hawaii, 171 pp. (2 vole).
MCFARLANE, J. A. 1982. Cassava storage. Part 2: Storage of dried cassava products. Tropical Science, 24, 205-236.
MONTALDO, A. 1967. Bibliograf�a de ra�ces y tub�rculos tropicales. Revista del Facultad Agronomico, Universidad Central de Venezuela, 13, 152-270; 515-516; 528-533; 543-545.
MONTALDO, A. 1972. Yuca o Mandioca. Cultivo de ra�ces y tub�rculos tropicales, pp. 51 - 136. Lima, Peru: Instituto Interamericano de Ciencias Agricolas de la OEA, 284 pp
NARTEY, F. 1978. Manihot esculenta (cassava). Cyanogenesis, ultrastructure and seed germination. Copenhagen, Denmark: Munksgaard: 262 pp.
NESTEL, B. and MACINTYRE, R. (eds). 1973. Chronic cassava toxicity:
Proceedings of an interdisciplinary workshop (London, 1973), IDRC-010e. Ottawa, Canada: International Development Research Centre, 162 pp.
NITIS, I. M. and SUARNA, M. 1977. Undersowing cassava with stylo grown under coconut. Proceedings of the 4th Symposium of the International
Society for Tropical Root Crops (Colombia, 1976), IDRC-080e (Cock, J., MacIntyre, R. and Graham, M., eds), pp. 98-103. Ottawa, Canada: International Development Research Centre, 277 pp.
NWEKE, F. I. 1981. Consumption patterns and their implications for research and production in tropical Africa. Tropical Root Crops: Research Strategies for the 1980s: Proceedings of the 1st Triennial Root Crops Symposium of the international Society for Tropical Root Crops - Africa Branch (Nigeria, 1980), IDRC-163e (Terry, E. R., Oduro, K. A. and Caveness, F., eds), pp. 88-94. Ottawa, Canada: International Development Research Centre, 279 pp.
OKE, D. L. 1968. Cassava as food in Nigeria. World Review of Nutrition and Dietetics, 9, 227-250.
PHILLIPS, T. P. 1973. Cassava utilisation and potential markets, IDRC-020e. Ottawa, Canada: International Development Research Centre, 182 pp.
PRAMANIK, A. 1971. Prospects for tapioca cultivation and pelletisation in Malaysia. Planter, Kuala Lumpur, 47 (543), 240-246.
PURSEGLOVE, J. W. 1968. Manihot Mill. Tropical crops: Dicotyledons 1, pp. 171-180. London: Longmans, Green and Co. Ltd, 332 pp.
RICKARD, J. E. 1985. Physiological deterioration of cassava roots. Journal of the Science of Food and Agriculture, 36, 167-176.
RICKARD, J. E. and COURSEY, D. G. 1981. Cassava storage. Part 1: Storage of fresh cassava roots. Tropical Science, 23, 1-32.
ROBERTS, A. T., TAYLOR, H. and MARGAI, M. 1983. Gari processing in Sierra Leone: an economic appraisal. Abstracts of the 6th Symposium of the International Society for Tropical Root Crops (Peru, 1983), p. 58. Lima, Peru: International Potato Center, 113 pp.
ROGERS, D. J. 1965. Some botanical and ethnological considerations of Manihot esculenta. Economic Botany, 19, 369-377.
ROGERS, D. J. and APPAN, S. G. 1973. Manihot, Manihotoides (Euphorbiaceae). Flora Neotropica, Monograph, No. 13, New York: Hafner Press, 272 pp.
SINGH, K. K. and MATHUR, P. B. 1953. Cold storage of tapioca roots. Bulletin of the Central Food Technological Research Institute (Mysore), 2, 181-182.
STRASSER, J., ABBOTT, J. A. and BATTEY, R. F. 1970. Process enriches cassava with protein. Food Engineering, 42 (5), 112; 114-116.
SUBRAMANYAM, H. and MATHUR, P. B. 1956. Effect of a fungicidal wax coating on the storage behaviour of tapioca roots. Bulletin of the Central Food Technological Research Institute (Mysore), 5, 110-111.
TERRY, E. R. and HAHN, S. K. 1982. Increasing and stabilizing cassava and sweet-potato productivity by disease resistance and crop hygiene. Root crops in Eastern Africa: Proceedings of a Workshop (Rwanda, 198O), IDRC-177e, pp. 47-52. Ottawa, Canada: International Development Research Centre, 128 pp. (Plant Breeding Abstracts, 53, 2340).
THAMPAN, A. K. 1979. Cassava. Trichur, Kerala, India: Kerala Agricultural University, 242 pp.
TORO, J. C. and ATTLEE, C. B. 1982. Practicas agronomicas pare la producci�n de yuca; una revisi�n de la literature. Yuca, Investigaci�n, Producci�n y Utilisaci�n (Dominguez, C. E., ed.), pp. 367-391. Cali, Colombia: Centro Internacional de Agricultura Tropical, 659 pp.
VARGHESE, G., THAMBIRAJAH, J. J. and WONG, F. M. 1977. Protein enrichment of cassava by fermentation with microfungi and the role of natural nitrogenous supplements. Proceedings of the 4th Symposium of the International Society for Tropical Root Crops (Colombia, 1976), IDRC-080e (Cock, J., MacIntyre, R. and Graham, M., eds), pp. 250-255. Ottawa, Canada: International Development Research Centre, 277 pp.
WALTERS, P. R. 1983. Cassava's markets - where next? World Crops, 35, 130-134.
WILLIAMS, C. N. 1972. Growth and productivity of tapioca (Manihot utilissima): crop ratio, spacing and yield. Experimental Agriculture, 8, 15-23.