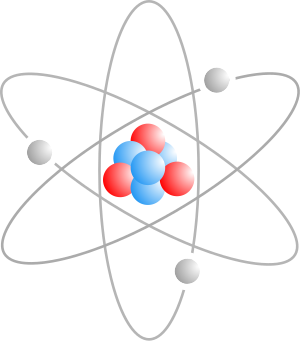
An atom is the smallest particle that comprises a chemical element. An atom consists of an electron cloud that surrounds a dense nucleus. This nucleus contains positively charged protons and electrically neutral neutrons, whereas the surrounding cloud is made up of negatively charged electrons. When the number of protons in the nucleus equals the number of electrons, the atom is electrically neutral; otherwise it is an ion and has a net positive or negative charge. An atom is classified according to its number of protons and neutrons: the number of protons determines the chemical element and the number of neutrons determines the isotope of that element. The concept of the atom as an indivisible component of matter was first proposed by early Indian and Greek philosophers. In the 17th and 18th centuries, chemists provided a physical basis for this idea by showing that certain substances could not be further broken down by chemical methods. During the late 19th and the early 20th centuries, physicists discovered subatomic components and structure inside the atom, thereby demonstrating that the 'atom' was not indivisible. The principles of quantum mechanics, including the wave–particle duality of matter, were used to successfully model the atom.[1][2]